|
|
| (143 intermediate revisions not shown) |
| Line 3: |
Line 3: |
| | <html><center><img src="https://static.igem.org/mediawiki/2010/0/00/TU_Delft_project_navigation.jpg" usemap="#projectnavigation" border="0" /></center><map id="projectnavigation" name="projectnavigation"><area shape="rect" alt="Characterization" title="" coords="309,3,591,45" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/characterization" target="" /><area shape="rect" alt="Results" title="" coords="609,3,891,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/results" target="" /><area shape="rect" alt="Parts" title="" coords="9,3,290,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/parts" target="" /></map></html> | | <html><center><img src="https://static.igem.org/mediawiki/2010/0/00/TU_Delft_project_navigation.jpg" usemap="#projectnavigation" border="0" /></center><map id="projectnavigation" name="projectnavigation"><area shape="rect" alt="Characterization" title="" coords="309,3,591,45" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/characterization" target="" /><area shape="rect" alt="Results" title="" coords="609,3,891,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/results" target="" /><area shape="rect" alt="Parts" title="" coords="9,3,290,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/parts" target="" /></map></html> |
| | ==Alkane Degradation Results & Conclusions== | | ==Alkane Degradation Results & Conclusions== |
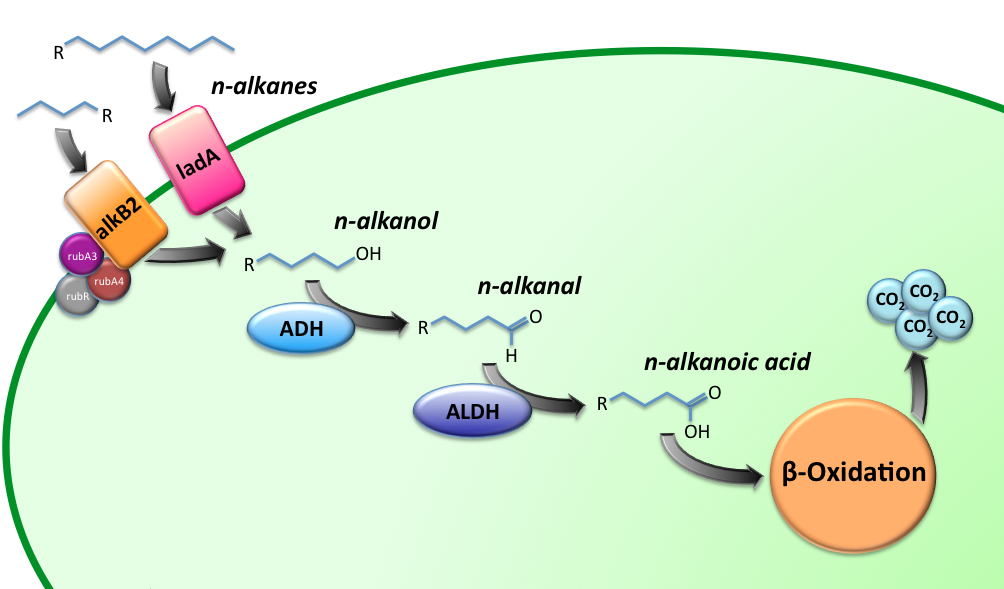
| - | [[Image:TUDelft_Alkane_degradation_route.png|600px|thumb|center|'''Figure 1''' – Schematic description of the alkane degradation pathway with the corresponding genes.]] | + | [[Image:TUDelft_Alkane_degradation_route.png|600px|thumb|center|'''Figure 1.''' – Schematic description of the alkane degradation pathway with the corresponding genes.]] |
| | | | |
| - | ===Characterization of the alkane hydroxylase system=== | + | ===[https://2010.igem.org/Team:TU_Delft/Project/alkane-degradation/results/alkane_hydroxylase Characterization of the alkane hydroxylase system (AlkB2-system)]=== |
| - | ====Growth analysis====
| + | |
| - | We attempted to culture our recombinant AH-carrying ''E.coli'' K12 strains ([http://partsregistry.org/Part:BBa_K398014 BBa_K398014]) on 1% v/v octanol or 1% v/v dodecane. Negligible growth was observed.
| + | |
| | | | |
| - | ====Resting-cell assays====
| |
| - | The [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Resting-cell_assays_for_E.coli resting cell assays] were performed on our recombinant AH-carrying ''E.coli'' K12 cells ([http://partsregistry.org/Part:BBa_K398014 BBa_K398014]). 100 micromoles of octane was added to 6 mL of growth-stalled cells (1.5 mg cell dry weight total) and incubated at 37 degrees with shaking o/n. The organic phase was [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Ethyl_acetate_extraction_protocol extracted] using EtOAc and analysed by [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=General_gas_chromatography_program_for_alkanes_and_alkanols gas chromatography].
| |
| | | | |
| - | The following are examples of typical chromatographs obtained:
| |
| | | | |
| - | <html><span style="display:block;width:100%;clear:both;"> </span></html>
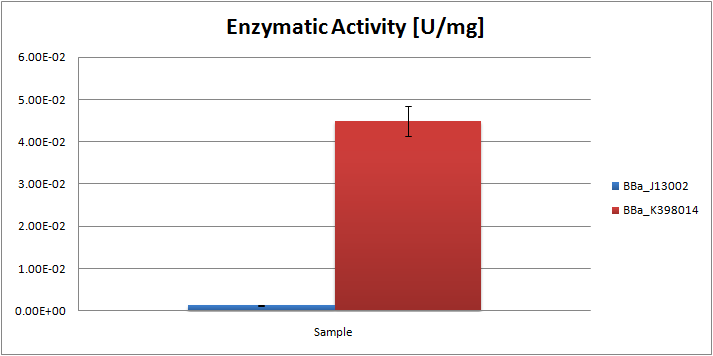
| + | From the ratios hexadecane/undecane obtained from GC chromatograms, we may conclude that the samples obtained from the ''E.coli'' strain, carrying the AH system, contain relatively less octane than the control strain. By comparing peak ratios we were able to estimate the specific enzymatic activity of the system, which was found to be 0.045 U/mg. |
| - | [[Image:TUDelft_J13-AH.png|400px|thumb|left|Chromatograph of the negative control for the resting cell assay of the alkane hydroxylase system. The negative control consisted of ''E.coli'' K12 carrying BBa_J13002 in pSB1A2.]]
| + | |
| | | | |
| - | [[Image:014 AH.png|400px|left|thumb|Chromatograph of ''E.coli'' K12 strain carrying the AH system.]] | + | For more information about our findings, read the [[Team:TU_Delft/Project/alkane-degradation/results/alkane_hydroxylase|detailed alkane hydroxylase results]] page. |
| - | <html><span style="display:block;width:100%;clear:both;"> </span></html>
| + | |
| | | | |
| - | The peaks shown in the chromatographs belong to the following compounds:
| + | [[Image:TUDelft_AlkB2_Total.png|600px|thumb|center|'''Figure 2.''' Enzyme activity [U/mg total protein] of alkane hydroxylase system as compared to the negative control ''E.coli'' K12 strain]] |
| - | {| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1"
| + | |
| - | |<b>Retention time [min]</b> | + | |
| - | |<b>Compound</b> | + | |
| - | |- | + | |
| - | |~2.2
| + | |
| - | |Ethyl acetate (solvent)
| + | |
| - | |-
| + | |
| - | |~5.2
| + | |
| - | |Octane (substrate)
| + | |
| - | |-
| + | |
| - | |~11.8
| + | |
| - | |Undecane (internal standard, 0.1% v/v of solvent)
| + | |
| - | |}
| + | |
| | | | |
| - | The surface areas of the peaks correspond to the amount of molecules present in the sample. Due to the fact that many factors play a role in establishing an equilibrium between the aqueous and organic phase once the EtOAC is added, it's general procedure to add an internal standard to the EtOAc. In our experiment we decided to use a 0.1% vol/vol of undecane in EtOAc. The <b>ratio</b> between the surface areas of octane and undecane can now give an indication of the amount of octane still present in the sample and be very useful for comparisons between chromatographs.
| + | ===[https://2010.igem.org/Team:TU_Delft/Project/alkane-degradation/results/LadA Characterization of the long-chain alkane monooxygenase (LadA)]=== |
| | | | |
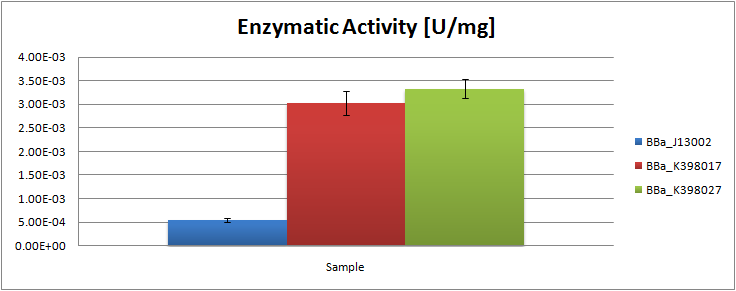
| - | The following table contains the surface areas found for the peaks of octane and undecane for each obtained chromatograph: | + | The analysis of the obtained GC graphs allowed us to estimate the enzymatic activity. We observed a significant increase in enzyme activity in the strains carrying the ladA protein generator compared to the negative control strain. The highest enzyme activity value was found to be 3.33E-03 U/mg protein. In order to know more about the characterization of this system, read the [[Team:TU_Delft/Project/alkane-degradation/results/LadA|detailed LadA results]] page. |
| | | | |
| - | {| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1"
| + | [[Image:TUDelft_LadA Total.png|600px|thumb|center|'''Figure 3.''' Enzyme activity [U/mg lysate] of the alkane monooxygenase LadA as compared to the negative control ''E.coli'' K12 strain]] |
| - | |<b>Strain</b> | + | |
| - | |<b>Octane surface area</b> | + | |
| - | |<b>Undecane surface area</b> | + | |
| - | |<b>Ratio octane/undecane</b>
| + | |
| - | |-
| + | |
| - | |''E.coli'' K12 negative control | + | |
| - | |17903555
| + | |
| - | |19939270
| + | |
| - | |0.898
| + | |
| - | |-
| + | |
| - | |''E.coli'' K12 014#2
| + | |
| - | |17426721
| + | |
| - | |24262069
| + | |
| - | |0.719
| + | |
| - | |-
| + | |
| - | |''E.coli'' K12 014#7
| + | |
| - | |7824594
| + | |
| - | |17320861
| + | |
| - | |0.452
| + | |
| - | |}
| + | |
| | | | |
| - | ===LadA=== | + | ===[https://2010.igem.org/Team:TU_Delft/Project/alkane-degradation/results/ADH Characterization of Alcohol Dehydrogenase (ADH)]=== |
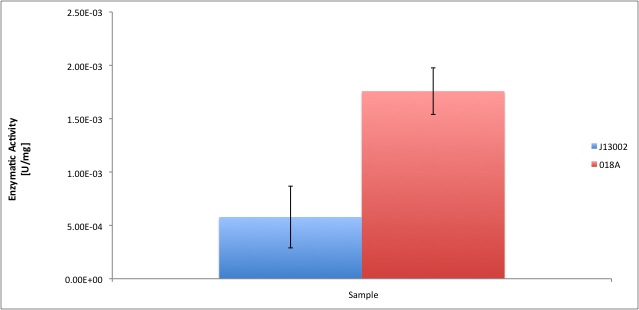
| | + | According to our results, the ''E. coli'' cell extract has a dodecanol-1 dehydrogenase activity of 9.64e-12 kat/mg (0.58 mU/mg); whereas our recombinant strain expressing the Biobrick [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] has an activity of 2.93e-11 kat/mg (1.76 mU/mg), an improvement of 2-fold compared to the wild type activity; which also means 3% of the activity of the positive control ''Pseudomonas putida''. |
| | | | |
| - | ===Characterization of the Alcohol DeHydrogenase (ADH) system===
| + | If you are interested in knowing more about our findings, read the [[Team:TU_Delft/Project/alkane-degradation/results/ADH|detailed ADH results]] page. |
| | | | |
| - | =====Growth on alcohols as sole carbon source=====
| + | [[Image:TUDelftADH_final.jpg|600px|thumb|center|'''Figure 4.''' Comparison between E. coli ADH activity and our recombinant strain. ]] |
| - | *As we described before, we tried to grow our strain ''Escherichia coli'' 018A ([http://partsregistry.org/Part:BBa_K398018 BBa_K398018] on the plasmid pSB1A2) on the alkanols Octanol-1 and Dodecanol-1 (1% V/V on M9 medium without any other carbon source). No growth was observed after 48 hours. We abandoned this experiment after this result.
| + | |
| | | | |
| - | =====Resting cell assays===== | + | ===[https://2010.igem.org/Team:TU_Delft/Project/alkane-degradation/results/ALDH Characterization of Aldehyde Dehydrogenase (ALDH)]=== |
| - | *We grew ''Escherichia coli'' 018A and ''Escherichia coli'' negative control (Biobrick BBa_J13002 on plasmid pSB1A2 ) in 50 mL of M9 medium with glucose and CAS aminoacids.
| + | |
| - | *The cells were harevested when the O.D. at 600nm was around 0.3; they were spun down at 4000 rpm, for 10 min at 4ºC. And the resting cell assays were prepared according to the [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Resting-cell_assays_for_E.coli standard protocol]
| + | |
| | | | |
| - | {| style="color:black; background-color:white;" cellpadding="2" cellspacing="0" border="1";
| + | Our results suggest that the recombinant strains ''E. coli'' 029A and ''E. coli'' 030A functionally express our biobricks. The expression of ALDH under the promoter-rbs combination [http://partsregistry.org/Part:BBa_J13002 BBa_J23100]-[http://partsregistry.org/Part:BBa_J13002 BBa_J61117] increases the dodecanal dehydrogenase activity in ''E. coli'' cell extracts 2-fold; whereas the expression of the same protein using the part [http://partsregistry.org/Part:BBa_J13002 BBa_J13002] as promoter-rbs combo increases the same activity 3-fold. |
| - | |'''Strain'''
| + | |
| - | |'''O.D.'''
| + | |
| - | |-
| + | |
| - | |J13002
| + | |
| - | |0.327
| + | |
| - | |-
| + | |
| - | |018A
| + | |
| - | |0.338
| + | |
| - | |}
| + | |
| - |
| + | |
| - | *After an overnight incubation at 37ºC, the organic phase was extracted using 3 mL of ethyl acetate (dodecane was used as internal standard). We tried to determine production of alkanals or alkanoic acids by Gas Chromatography measurements. The chromatograms are shown below:
| + | |
| | | | |
| - | [[Image:TUDelftADH_chrom.jpg|750px|thumb|center|Typical chromatograms obtained after the resting cell assays: (A) Blank, (B) Negative control which is E. coli K12, (C) E. coli 018A (our recombinant strain) and (D) standard of the expected product.]] | + | Moreover, the enzymatic activities measured for the constructs [http://partsregistry.org/Part:BBa_K398029 BBa_K398029] and [http://partsregistry.org/Part:BBa_K398030 BBa_K398030] were equivalent to 33.98% and 42.01% of the ''Pseudomonas putida'' aldehyde dehydrogenase activity, respectively. |
| | | | |
| | + | If you want to know more about of our findings, read the [[Team:TU_Delft/Project/alkane-degradation/results/ALDH|detailed ALDH results]] page. |
| | | | |
| - | *According to our results there was no degradation of octanol-1 ='( , after this experiment the protocol was abandoned. We think that the cells lack of transporters for long-chain alcohols, we preferred to check the cell extract in order to know if there was any biological activity in the cytoplasma.
| + | It is worthy to mention that our part expresses the protein in a lower amount, meaning that cells express a highly active protein. Thus the cellular resources are spent in a more efficient way than in the strain that overproduces ALDH. |
| | | | |
| - | =====NADH production in cell extracts=====
| + | [[Image:TUDelftALDH_final.jpg|600px|thumb|center|'''Figure 5.''' Comparison of ALDH activities in the different strains tested in this study]] |
| - | *'''EXPERIMENT 1'''
| + | |
| - | Cells were cultured in 50mL of LB medium and harvested when the O.D. 600nm of the culture was around 0.6. Two different cultures of the recominant strain and the negative control were prepared.
| + | |
| - | {| style="color:black; background-color:white;" cellpadding="2" cellspacing="0" border="1"
| + | |
| - | |Strain
| + | |
| - | |O.D. 600nm
| + | |
| - | |-
| + | |
| - | |J13002 (1)
| + | |
| - | |0.654
| + | |
| - | |-
| + | |
| - | |J13002 (2)
| + | |
| - | |0.621
| + | |
| - | |-
| + | |
| - | |018A (1)
| + | |
| - | |0.615
| + | |
| - | |-
| + | |
| - | |018A (2)
| + | |
| - | |0.580
| + | |
| - | |}
| + | |
| - | Cytoplasmic proteins were extracted using our standard [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Preparing_cell_lysates_for_enzyme_kinetics_measurements protocol]. And a standard curve for protein quantification was prepared.
| + | |
| - | [[Image:TUDelftADH_bradford.jpg|600px|thumb|center|Standard curve for total protein quantification.]] | + | |
| - | The total protein of each sample was quantified using 20uL of cell extract, the results are shown below.
| + | |
| - | {| style="color:black; background-color:white;" cellpadding="4" cellspacing="0" border="1"
| + | |
| - | |Sample
| + | |
| - | |'''O.D. 562 nm''' | + | |
| - | |'''Total ug of protein'''
| + | |
| - | |'''ug/uL'''
| + | |
| - | |-
| + | |
| - | |J13002(1A)
| + | |
| - | |0.135
| + | |
| - | |10.393
| + | |
| - | |1.0393
| + | |
| - | |-
| + | |
| - | |J13002 (1B)
| + | |
| - | |0.135
| + | |
| - | |10.393
| + | |
| - | |1.0393
| + | |
| - | |-
| + | |
| - | |J13002 (2A)
| + | |
| - | |0.128
| + | |
| - | |9.7363
| + | |
| - | |0.9736
| + | |
| - | |-
| + | |
| - | |J13002 (2B)
| + | |
| - | |0.129
| + | |
| - | |9.8302
| + | |
| - | |0.9830
| + | |
| - | |-
| + | |
| - | |018A (1A)
| + | |
| - | |0.097
| + | |
| - | |6.8275
| + | |
| - | |0.6827
| + | |
| - | |-
| + | |
| - | |018A (1B)
| + | |
| - | |0.091
| + | |
| - | |6.2645
| + | |
| - | |0.6264
| + | |
| - | |-
| + | |
| - | |018A (2A)
| + | |
| - | |0.092
| + | |
| - | |6.3583
| + | |
| - | |0.6358
| + | |
| - | |-
| + | |
| - | |018A (2B)
| + | |
| - | |0.092
| + | |
| - | |6.3583
| + | |
| - | |0.6358
| + | |
| - | |}
| + | |
| - | The alcohol dehydrogenase activity was measured using the standard [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Alcohol.2FAldehyde_dehydrogenase_activity_assays protocol] and Dodecanol-1 as substrate. You can download our raw data by clicking on the link: [[ Image:TUDelft_ADH_raw.xls]]
| + | |
| - | After the data treatment, the results that we obtained were the following:
| + | |
| - | [[Image:TUDelftADH_pH95.jpg|400px]]
| + | |
| - | [[Image:TUDelftADH_pH8.jpg|400px]]
| + | |
| - | | + | |
| - | *All the enzyme activities were normalized to the TOTAL amount of protein in the cell extract. The results are shown in katal per mg of protein in the cell extract and Enzymatic Units per mg of protein in the cell extract.
| + | |
| - | | + | |
| - | <html>
| + | |
| - | <table>
| + | |
| - | <tr><td></td><td colspan=4 style="text-align: center;"><b>pH=9.5</b></td><td colspan=4 style="text-align: center;"><b>pH=8</b></td></tr>
| + | |
| - | <tr><td> </td><td colspan=2 style="text-align: center;"><b>Heat</b></td><td colspan=2 style="text-align: center;"><b>Native</b></td><td colspan=2 style="text-align: center;"><b>Heat</b></td><td colspan=2 style="text-align: center;"><b>Native</b></td></tr>
| + | |
| - | <tr><td> </td><td>J13002</td><td>018A</td><td>J13002</td><td>018A</td><td>J13002</td><td>018A</td><td>J13002</td><td>018A</td></tr>
| + | |
| - | <tr><td><b>kat/mg</b></td><td>6,156E-12</td><td>1,941E-11</td><td>1,053E-11</td><td>2,806E-11</td><td>3,211E-12</td><td>4,496E-11</td><td>1,302E-11</td><td>2,638E-11</td></tr>
| + | |
| - | <tr><td><b>U/mg</b></td><td>3,694E-04</td><td>1,091E-03</td><td>6,317E-04</td><td>1,694E-03</td><td>1,927E-04</td><td>2,139E-03</td><td>7,811E-04</td><td>1,608E-03</td></tr>
| + | |
| - | <tr><td><b>stdev</b></td><td>1,157E-12</td><td>4,579E-12</td><td>1,343E-12</td><td>9,961E-13</td><td>6,395E-12</td><td>2,331E-11</td><td>6,511E-13</td><td>9,961E-13</td></tr>
| + | |
| - | <tr><td><b>Improvement</b></td><td>-</td><td>215,34%</td><td>-</td><td>166,51%</td><td>-</td><td>1299,87%</td><td>-</td><td>166,51%</td></tr>
| + | |
| - | <tr><td><b>ttest</b></td><td>-</td><td>0,9963</td><td>-</td><td>1,0000</td><td>-</td><td>0,9988</td><td>-</td><td>1,000</td></tr>
| + | |
| - | </table>
| + | |
| - | </html>
| + | |
| - | | + | |
| - | You can download our results file here: [[ Image:TUDelft_ADH_results.xls]]
| + | |
| - | Maybe you are not familiar with the term "katal", officially the katal is the STANDARD UNIT FOR CATALYTIC ACTIVITY in the International System of Units; the katal is defined as the amount of enzyme that converts 1 mol of substrate each second. [http://www.clinchem.org/cgi/content/full/48/3/586 Click here] to know more about it, and because people are not that familiar with the term, we report the activities in U/mg. One enzyme activity unit is defined as the amount of protein that converts 1 umol of substrate each minute.
| + | |
| - | T-tests were performed in order to show that there is a difference between E. coli and our recombinant strain; a result for t-test of 1 means that both samples are statistically different; whereas a t-test of nearly 0 means that samples belong to the same group (no difference between both). Our level of significance is 0.025, in two tailed test. That means that if our t-test result is 0.95 we conclude that both samples are statistically different, whereas 0.949 or lower means that both samples are not statistically different.
| + | |
| - | | + | |
| - | | + | |
| - | *'''EXPERIMENT 2'''
| + | |
| - | We ran a second experiment at pH 9.5, with two other strains that seemed to be positive in the colony PCR test. In this test we also added two extra strains of E. coli in order to have more data for the statistical analysis, the strains used were J13002 and 331 ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K398331 BBa_K398331] in pSB1A2). We followed the same procedure as in the experiment 1, for this experiment we also included a positive control which was cell extracts from a culture of ''Pseudomonas putida'' growing on octane as sole carbon source, the cells were inoculated the night before and they were harvested when the O.D. at 600 nm was 0.608; the results obtained were the following:
| + | |
| - | | + | |
| - | <table class="tableizer-table">
| + | |
| - | <tr class="tableizer-firstrow"><th></th><th>kat/mg</th><th>U/mg</th><th>STDEV</th><th>Improvement</th><th>T-test</th><th>Relative to putida</th><th> </th></tr> <tr><td>E. Coli</td><td>8.75E-12</td><td>5.25E-04</td><td>8.18987E-12</td><td>-</td><td>-</td><td>0.90%</td><td> </td></tr> <tr><td>018A-2</td><td>9.30E-12</td><td>5.58E-04</td><td>1.84877E-12</td><td>6.26%</td><td>0.4708</td><td>0.96%</td><td> </td></tr> <tr><td>018A-3</td><td>3.05E-11</td><td>1.83E-03</td><td>6.27802E-12</td><td>249.14%</td><td>0.9808</td><td>3.15%</td><td> </td></tr> <tr><td>018A-4</td><td>8.26E-12</td><td>4.96E-04</td><td>1.8256E-12</td><td>-5.59%</td><td>0.4053</td><td>0.85%</td><td> </td></tr> <tr><td>P. Putida</td><td>9.69E-10</td><td>5.82E-02</td><td>2.09109E-10</td><td>-</td><td>0.9883</td><td>100.00%</td><td></td></tr></table>
| + | |
| - | | + | |
| - | According to these results, the other two strains (018A-2 and 018A-4) do not functionally express the part BBa_K398018. However, these results confirm our findings from the first experiment. You can download our result in this link: [[ Image:TUDelft_ADH_results2.xls]] You can also find a summary in this file:[[ Image:TUDelft_ADH_summary.xls]]
| + | |
| - | | + | |
| - | ==== Conclusions ====
| + | |
| - | | + | |
| - | [[Image:TUDelftADH_final.jpg|600px]]
| + | |
| - | | + | |
| - | According to our results, a normal ''E. coli'' cell extract has a dodecanol-1 dehydrogenase activity of 9.64e-12 kat/mg (0.58 mU/mg); whereas our recombinant strain 018A has an activity of 2.93e-11 kat/mg (1.76 mU/mg). According to our analysis, the enzymatic activities of both strains are statistically different at confidence level of 0.95 which means that the part [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] increases 2 times the alcohol dehydrogenase activity in the cell extract. We can conclude from our data that the parts [http://partsregistry.org/Part:BBa_K398005 BBa_K398005] and the part [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] have biological activity; particularly when we used [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] the enzyme activity of ''E. coli'' cell extracts became equivalent to 3% of the ''in vitro'' activity of the positive control (''Pseudomonas putida''). A comparison of both activities maybe it is not interesting from the functional point of view, since those differences could be related to substrate affinity or discrepancies in the optimal pH.
| + | |
| - | However, we think that from this comparison of both ''in vitro'' activities we can suggest future teams interested in our part [http://partsregistry.org/Part:BBa_K398005 BBa_K398005] to use a stronger promoter-rbs combination; maybe by increasing the amount of protein produced it is possible to get higher ''in vitro'' and ''in vivo'' activities. Nevertheless, we have to stress that the ''in vivo'' activity of the part [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] is still unknown for us, maybe there is a lack of long-chain alcohol transporter proteins or it is necessary to over-express this part in order to see/measure it.
| + | |
| - | | + | |
| - | ===Characterization of the ALdehyde DeHydrogenase system===
| + | |
| - | | + | |
| - | =====Growth on alcohols as sole carbon source=====
| + | |
| - | As we described before, we tried to grow our recombinant strains ''Escherichia coli'' 029A ([http://partsregistry.org/Part:BBa_K398029 BBa_K398029] on the plasmid pSB1A2) and ''Escherichia coli'' 030A ([http://partsregistry.org/Part:BBa_K398030 BBa_K398030] on the plasmid pSB1A2) on Octanal and Dodecanal. No growth was observed after 48 hours. We abandoned this experiment after this result.
| + | |
| - | | + | |
| - | =====Resting cell assays=====
| + | |
| - | *We grew 'Escherichia coli'' 029A, ''Escherichia coli'' 030Aand ''Escherichia coli'' negative control (Biobrick BBa_J13002 on plasmid pSB1A2 ) in 50 mL of M9 medium with glucose and CAS aminoacids.
| + | |
| - | *The cells were harevested when the O.D. at 600nm was around 0.3; they were spun down at 4000 rpm, for 10 min at 4ºC. And the resting cell assays were prepared according to the [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Resting-cell_assays_for_E.coli standard protocol]
| + | |
| - | | + | |
| - | {| style="color:black; background-color:white;" cellpadding="2" cellspacing="0" border="1";
| + | |
| - | |'''Strain'''
| + | |
| - | |'''O.D.'''
| + | |
| - | |-
| + | |
| - | |J13002
| + | |
| - | |0.327
| + | |
| - | |-
| + | |
| - | |029A
| + | |
| - | |0.340
| + | |
| - | |-
| + | |
| - | |030A
| + | |
| - | |0.330
| + | |
| - | |}
| + | |
| - |
| + | |
| - | '''Note''': This experiment was run in parallel with the resting cell assays for 018A, the negative control culture of the strain J13002 used was the same for both experiments.
| + | |
| - | | + | |
| - | *After an overnight incubation at 37ºC with the substrate (octanal), the organic phase was extracted using 3 mL of ethyl acetate (dodecane was used as internal standard). We tried to determine production of alkanoic acids by Gas Chromatography measurements. The chromatograms are shown below:
| + | |
| - | | + | |
| - | [[Image:TUDelftALDH_chrom.jpg|750px|thumb|center| Typical chromatograms obtained after the resting cell assays: (A) Blank, (B) Negative control which is E. coli K12, (C) E. coli 029A (our recombinant strain) and (D) E. coli 029A (our recombinant strain).]]
| + | |
| - | | + | |
| - | | + | |
| - | *According to our results there was a peak reduction for octanal, however we were worried because of the fact that we didn't see the peak of the product (dodecanoic acid) appearing. It could be that the cells are just storing the product inside the cell. The experiment required to much time in order to get more results: the extractions, preparation of triplicates, cultures, cell suspensions... etc. it was a work that would require an effort of all the team for a couple of weeks. We decided to go for something easier and faster to measure; since we had some experience with the NADH determinations that was our choice.
| + | |
| - | | + | |
| - | ==== NADH production in cell extracts====
| + | |
| - | Cells were cultured in 50mL of LB medium and harvested when the O.D. 600nm of the culture was between 0.5-0.8. Two different cultures of each recominant strain and the negative control were prepared.
| + | |
| - | | + | |
| - | {| style="color:black; background-color:white;" cellpadding="2" cellspacing="0" border="1"
| + | |
| - | |Strain
| + | |
| - | |O.D. 600nm
| + | |
| - | |-
| + | |
| - | |J13002 (1)
| + | |
| - | |0.825
| + | |
| - | |-
| + | |
| - | |J13002 (2)
| + | |
| - | |0.774
| + | |
| - | |-
| + | |
| - | |029A (1)
| + | |
| - | |0.746
| + | |
| - | |-
| + | |
| - | |029A (2)
| + | |
| - | |0.753
| + | |
| - | |-
| + | |
| - | |030A (1)
| + | |
| - | |0.532
| + | |
| - | |-
| + | |
| - | |030A (2)
| + | |
| - | |0.532
| + | |
| - | |}
| + | |
| - | Cytoplasmic proteins were extracted using our standard [https://2010.igem.org/Team:TU_Delft#Preparing_cell_lysates_for_enzyme_kinetics_measurements protocol]. And a standard curve for protein quantification was prepared.
| + | |
| - | | + | |
| - | [[Image:TUDelftALDH_bradford.jpg|600px|thumb|center|Standard curve for total protein quantification.]]
| + | |
| - | | + | |
| - | The total protein of each sample was quantified using 20uL of cell extract, the results are shown below.
| + | |
| - | | + | |
| - | <table class="tableizer-table">
| + | |
| - | <tr class="tableizer-firstrow"><th></th><th>Total protein [ug]</th><th>ug/uL</th></tr> <tr><td>J1300</td><td>31.108</td><td>1.555</td></tr> <tr><td>029A (1)</td><td>19.974</td><td>0.999</td></tr> <tr><td>030A (1)</td><td>12.610</td><td>0.630</td></tr> <tr><td>029A (2)</td><td>12.854</td><td>0.782</td></tr> <tr><td>030 (2)</td><td>18.578</td><td>0.929</td></tr> <tr><td>P. Puitda</td><td>9.783</td><td>0.489</td></tr></table>
| + | |
| - | | + | |
| - | | + | |
| - | The aldehyde dehydrogenase activity was measured using the standard [https://2010.igem.org/Team:TU_Delft#page=Notebook/protocols&anchor=Alcohol.2FAldehyde_dehydrogenase_activity_assays protocol] and Dodecanal as substrate.
| + | |
| - | | + | |
| - | After the data treatment, the results that we obtained were the following:
| + | |
| - | | + | |
| - | <table class="tableizer-table">
| + | |
| - | <tr class="tableizer-firstrow"><th></th><th> </th><th>J13002</th><th>029A (1)</th><th>029A (2)</th><th>030A (1)</th><th>030A (2)</th><th>P. putida</th></tr> <tr><td>Specific activity</td><td>kat/mg</td><td>8.604E-11</td><td>1.470E-10</td><td>2.853E-10</td><td>2.433E-10</td><td>2.912E-10</td><td>6.362E-10</td></tr> <tr><td> </td><td>U/mg</td><td>5.162E-03</td><td>8.820E-03</td><td>1.712E-02</td><td>1.460E-02</td><td>1.747E-02</td><td>3.817E-02</td></tr> <tr><td>Standard deviation</td><td> </td><td>1.778E-12</td><td>6.187E-12</td><td>4.026E-11</td><td>3.539E-11</td><td>4.319E-11</td><td>3.361E-11</td></tr> <tr><td>Relative to (J13002)</td><td> </td><td>100.00%</td><td>170.86%</td><td>331.59%</td><td>282.79%</td><td>338.46%</td><td>739.41%</td></tr> <tr><td>Relative activity (P. putida)</td><td> </td><td>13.52%</td><td>23.11%</td><td>44.84%</td><td>38.25%</td><td>45.78%</td><td>100.00%</td></tr> <tr><td>T-test vs J13002</td><td> </td><td>-</td><td>0.9827</td><td>0.9599</td><td>0.9764</td><td>0.9976</td><td>0.9976</td></tr> <tr><td>T-test vs P. putida</td><td> </td><td>0.9999</td><td>1.0000</td><td>0.9998</td><td>0.9974</td><td>1.0000</td><td>-</td></tr> <tr><td>Average activity [kat/mg]</td><td> </td><td>8.604E-11</td><td>2.161E-10</td><td> </td><td>2.673E-10</td><td> </td><td>6.362E-10</td></tr></table>
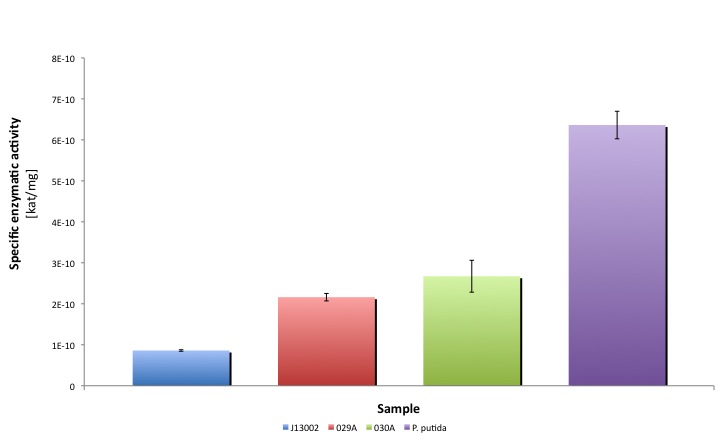
| + | |
| - | | + | |
| - | You can download a file with our raw data, results anda summary here: [[ Image:TUDelft_ALDH_results.xls]]
| + | |
| - | | + | |
| - | ==== Conclusion====
| + | |
| - | | + | |
| - | Our results suggest that the recombinant strains ''E. coli'' 029A and ''E. coli'' 030A functionally express our biobricks, we couldn't prove ''in vivo'' activity. However, it is clear from the statistical analysis performed that the expression of ... under the promoter-rbs combination increases the dodecanal dehydrogenase activity in E. coli cell extracts 2-fold; whereas the expression of the same protein using the part J13002 increases the same activity 3-fold. Moreover, the enzymatic activities measured are equivalent to % and % of the Pseudomonas putida aldehyde dehydrogenase activity.
| + | |
| - | | + | |
| - | | + | |
| - | [[Image:TUDelftALDH_final.jpg|600px]]
| + | |
| | | | |
| | | | |
| | + | ===References=== |
| | + | #Kato T. et al. "Gene cloning and characterization of an aldehyde dehydrogenase from long-chain alkane-degrading ''Geobacillus thermoleovorans'' B23" Extremophiles (2010) 14:33-39. |
| | + | #http://mbel.kaist.ac.kr/lab/research/protein_en1.html |
| | + | #Hoffmann F. and Rinas U. "Stress Induced by Recombinant Protein Production in ''Escherichia coli''" Advances in Biochemical Engineering/Biotechnology, 2004, Vol. 89/2004, pp. 73-92. |
| | | | |
| | <html><center><img src="https://static.igem.org/mediawiki/2010/0/00/TU_Delft_project_navigation.jpg" usemap="#projectnavigation" border="0" /></center><map id="projectnavigation" name="projectnavigation"><area shape="rect" alt="Characterization" title="" coords="309,3,591,45" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/characterization" target="" /><area shape="rect" alt="Results" title="" coords="609,3,891,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/results" target="" /><area shape="rect" alt="Parts" title="" coords="9,3,290,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/parts" target="" /></map></html> | | <html><center><img src="https://static.igem.org/mediawiki/2010/0/00/TU_Delft_project_navigation.jpg" usemap="#projectnavigation" border="0" /></center><map id="projectnavigation" name="projectnavigation"><area shape="rect" alt="Characterization" title="" coords="309,3,591,45" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/characterization" target="" /><area shape="rect" alt="Results" title="" coords="609,3,891,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/results" target="" /><area shape="rect" alt="Parts" title="" coords="9,3,290,44" href="https://2010.igem.org/Team:TU_Delft#page=Project/alkane-degradation/parts" target="" /></map></html> |
From the ratios hexadecane/undecane obtained from GC chromatograms, we may conclude that the samples obtained from the E.coli strain, carrying the AH system, contain relatively less octane than the control strain. By comparing peak ratios we were able to estimate the specific enzymatic activity of the system, which was found to be 0.045 U/mg.
The analysis of the obtained GC graphs allowed us to estimate the enzymatic activity. We observed a significant increase in enzyme activity in the strains carrying the ladA protein generator compared to the negative control strain. The highest enzyme activity value was found to be 3.33E-03 U/mg protein. In order to know more about the characterization of this system, read the detailed LadA results page.
Moreover, the enzymatic activities measured for the constructs [http://partsregistry.org/Part:BBa_K398029 BBa_K398029] and [http://partsregistry.org/Part:BBa_K398030 BBa_K398030] were equivalent to 33.98% and 42.01% of the Pseudomonas putida aldehyde dehydrogenase activity, respectively.
It is worthy to mention that our part expresses the protein in a lower amount, meaning that cells express a highly active protein. Thus the cellular resources are spent in a more efficient way than in the strain that overproduces ALDH.


 "
"