Team:EPF Lausanne/Project/Background
From 2010.igem.org
(→Structural information about P-proteins) |
(→Candidate effector molecules to block malaria propagation) |
||
| (21 intermediate revisions not shown) | |||
| Line 19: | Line 19: | ||
<div CLASS="EPFL_content"> | <div CLASS="EPFL_content"> | ||
= Immunotoxin = | = Immunotoxin = | ||
| - | + | [[Image:Immuno.png|right|150px|caption]] | |
The [http://partsregistry.org/Part:BBa_K320001 immunotoxin] is composed of two main parts. The first is a single-chain antibody fragment (scFv) directed to Pbs2l, a surface membrane protein of P. berghei ookinetes. This fragment is linked to the second part, a lytic peptide, Shiva-1. Shiva-1 is a synthetic peptide with an amino acid sequence similar, in terms of length (38 amino acids, charge distribution as well as hydrophobicity, to Cecropin B which is an antibacterial peptide. The purpose of the immunotoxin is to specifically target and lyse the parasite. | The [http://partsregistry.org/Part:BBa_K320001 immunotoxin] is composed of two main parts. The first is a single-chain antibody fragment (scFv) directed to Pbs2l, a surface membrane protein of P. berghei ookinetes. This fragment is linked to the second part, a lytic peptide, Shiva-1. Shiva-1 is a synthetic peptide with an amino acid sequence similar, in terms of length (38 amino acids, charge distribution as well as hydrophobicity, to Cecropin B which is an antibacterial peptide. The purpose of the immunotoxin is to specifically target and lyse the parasite. | ||
| Line 29: | Line 29: | ||
= The "P-proteins" = | = The "P-proteins" = | ||
| - | + | [[Image:P28.png|left|150px|caption]] [[Image:P25.png|left|150px|caption]] | |
P25 and P28 are a class of important proteins expressed on the membrane of different type of Plasmodium; we call this ensemble of evolutionary conserved proteins the P-proteins. They are mainly expressed on the mosquito-stage parasite (ookinete). | P25 and P28 are a class of important proteins expressed on the membrane of different type of Plasmodium; we call this ensemble of evolutionary conserved proteins the P-proteins. They are mainly expressed on the mosquito-stage parasite (ookinete). | ||
The ookinete has been intensively studied by scientists, looking for an ideal transmission-blocking vaccine target. | The ookinete has been intensively studied by scientists, looking for an ideal transmission-blocking vaccine target. | ||
| Line 44: | Line 44: | ||
The team decided to design Asaia to express a soluble form of these two P-proteins. It may seem contra-intuitive to provide more of these proteins (by expression in Asaia) that are essential for survival, migration, and development of the parasite. However, our hypothesis is based on the fact that these proteins over-expressed in a soluble form would compete with the parasite surface protein and prevent the interaction necessary to its transmission. | The team decided to design Asaia to express a soluble form of these two P-proteins. It may seem contra-intuitive to provide more of these proteins (by expression in Asaia) that are essential for survival, migration, and development of the parasite. However, our hypothesis is based on the fact that these proteins over-expressed in a soluble form would compete with the parasite surface protein and prevent the interaction necessary to its transmission. | ||
Because the functions of P25 and P28 are redundant, both proteins need to be inhibited or outcompeted to efficiently block the malaria transmission. | Because the functions of P25 and P28 are redundant, both proteins need to be inhibited or outcompeted to efficiently block the malaria transmission. | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:Wiktor1.png|right|250px|caption]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
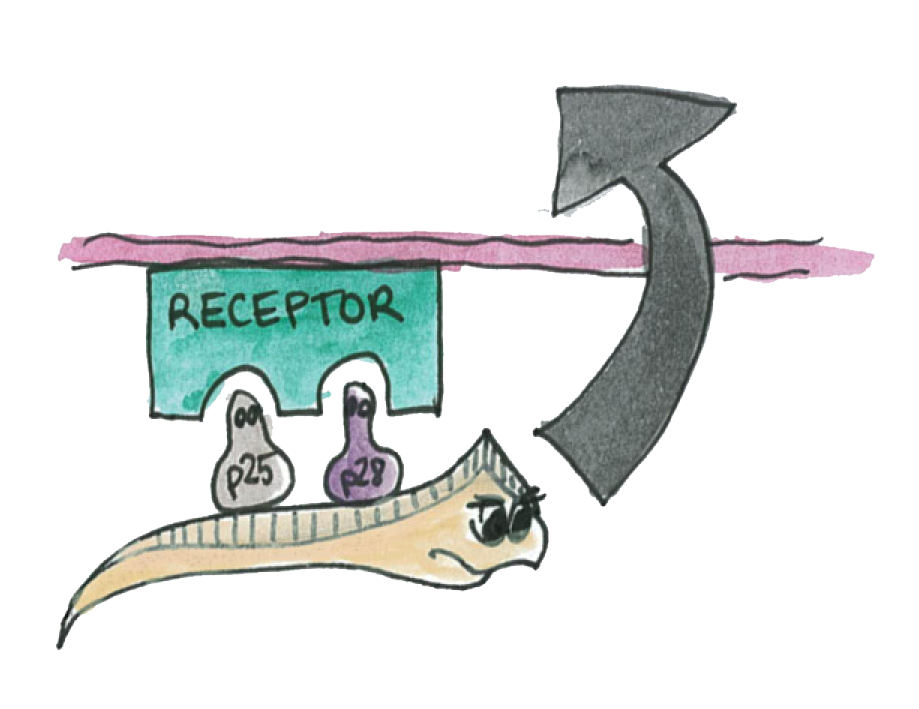
| + | As illustrated on the picture, the P-proteins mediate the transmission of the parasite through the epithelium. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:Wiktor3.png|left|250px|caption]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
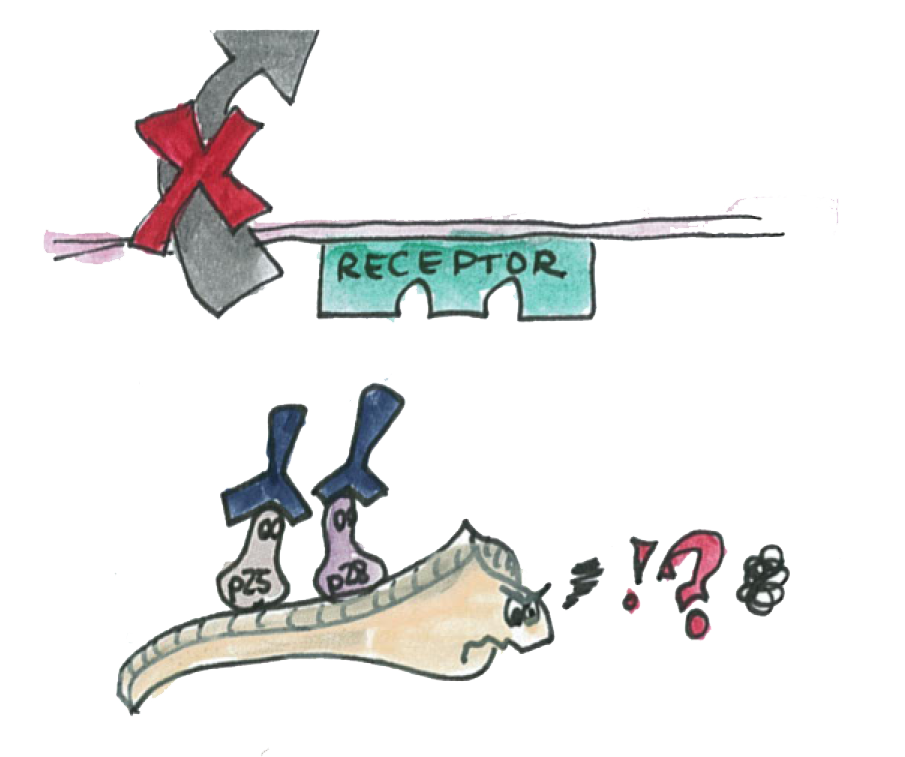
| + | We know that a double knockout of these proteins make it impossible for the parasite to traverse the epithelium. | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:Wiktor2.png|right|250px|caption]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | We also know that antibodies specific to the P-proteins also inhibit the parasite transmission. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:Wiktor4.png|left|250px|caption]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | However, if we are able to express the P-proteins by Asia in the mosquito's gut the P-protein interactions will be disrupted and the parasite will not we able to cross the epithelium. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
== Structural information about P-proteins == | == Structural information about P-proteins == | ||
| Line 55: | Line 111: | ||
An interesting feature of the X-ray crystal structure of the Pvs25 : the four EGF-like domains form a compact triangular prism. These prisms are arranged on layers of sheets in the crystal. P. falciparum P25 (Pfs25) likely assume a similar structure (see Figure 1 and crystal structure). These structures may play a role of protection of the parasite, cysteins play an important role to maintain this structure by making 11 and 10 disulfide bonds in the P25 and P28 protein respectively. | An interesting feature of the X-ray crystal structure of the Pvs25 : the four EGF-like domains form a compact triangular prism. These prisms are arranged on layers of sheets in the crystal. P. falciparum P25 (Pfs25) likely assume a similar structure (see Figure 1 and crystal structure). These structures may play a role of protection of the parasite, cysteins play an important role to maintain this structure by making 11 and 10 disulfide bonds in the P25 and P28 protein respectively. | ||
| - | [[Image:P-protein_sheet.jpeg|350px|center|bottom|thumb|bottom|'''Figure 1. '''Sheets of Pvs25 (Plasmodium vivax) in the crystal structure [ [[#References:|4]] ]]] | + | [[Image:P-protein_sheet.jpeg|350px|center|bottom|thumb|bottom|'''Figure 1. '''Sheets of Pvs25 (Plasmodium vivax) in the crystal structure [ [[#References:|4]]] ]] |
In order to find the protein, we attached a His tag to the P-proteins when we designed the primers to extract the proteins from the Plasmodium gDNA. We attached it to the C-terminus. The reason for that is that the C-terminus is involved in the attachment of the protein to the plasmodium outer membrane and thus should not e involved on the P-protein – P-protein and P-protein – Receptor interactions. This idea is supported by experiments with transmission-blocking antibodies. Indeed wee can see on the figure that the epitop of the transmission-blocking antibody is far away from the C-terminus. So we think that the addition of the His-tag does not compromise the functionalities of the proteins. | In order to find the protein, we attached a His tag to the P-proteins when we designed the primers to extract the proteins from the Plasmodium gDNA. We attached it to the C-terminus. The reason for that is that the C-terminus is involved in the attachment of the protein to the plasmodium outer membrane and thus should not e involved on the P-protein – P-protein and P-protein – Receptor interactions. This idea is supported by experiments with transmission-blocking antibodies. Indeed wee can see on the figure that the epitop of the transmission-blocking antibody is far away from the C-terminus. So we think that the addition of the His-tag does not compromise the functionalities of the proteins. | ||
| - | [[Image:Epitop.jpg|350px|center|bottom|thumb|bottom|'''Figure 2. '''Heavy chain (dark blue) of the transmission-blocking antibody binding the Pvs25 protein [4]]] | + | [[Image:Epitop.jpg|350px|center|bottom|thumb|bottom|'''Figure 2. '''Heavy chain (dark blue) of the transmission-blocking antibody binding the Pvs25 protein [ [[#References:|4]] ]]] |
Here is the X-ray crystal structure of P25: | Here is the X-ray crystal structure of P25: | ||
[http://www.pdb.org/pdb/explore/jmol.do?structureId=1Z1Y&bionumber=1 Crystal structure] | [http://www.pdb.org/pdb/explore/jmol.do?structureId=1Z1Y&bionumber=1 Crystal structure] | ||
| - | == | + | =Candidate effector molecules to block malaria propagation= |
| + | |||
| + | At the beginning of our project, we have conducted further research in order to find all possible ways to block the transmission of malaria. Here you can find some of the most interesting ideas: | ||
| + | |||
| + | *A lytic peptide called '''SB-37''' similar (in term of length and amino acids property) to Shiva-1 (the lytic peptide of our ordered immunotoxin) was used to kill P. falciparum in vitro [5]. SB-37 is a slightly modified version of a Cecropin, the Cecropin B [5], the sequence is in the paper [5]. | ||
| + | |||
| + | |||
| + | *'''Cecropins''' are peptides derived from the insect immune defence (a giant moth). Cecropin A was expressed in a simbiont of the bug Rhodnius prolixus in order to kill T. cruzi, a parasite that cause the Chagas disease [6]. More recently, It was also expressed in the mosquitoís gut (transgenic mosquitoes) to prevent the propagation of the malaria [7] (See [http://www.anaspec.com/products/product.asp?id=30699 Sequence]). Because of its hight homology the cecropins A and B, cecropin D could also be used (sequence: [7]). There exists 6 cecropins : cecropin A, Ö , F. | ||
| + | |||
| + | |||
| + | *The '''Glossina attacin''' is an antimicrobial peptide effective on both gram-negative bacteria and protozoa (Plasmodium is a protozoa!). It was expressed in a symbiont of the tsetse fly in order to kill the pasasite T.brucei; We should determine if it acts on Asaia, attacins kill E.Coli but [http://www.copewithcytokines.org/cope.cgi?key=Attacins do not act on many other gram-positive and gram-negative bacteria]. Sequence: [9]. | ||
| + | |||
| + | |||
| + | |||
| + | Other effectors molecules against Plasmodium are cited by Jacobs-Lorena and al. [10]: | ||
| + | |||
| + | *The '''salivary gland/midgut peptide (SM1)''', it is a dodecapeptide (sequence: [11]), It was found by testing a number of radom peptides, and it binds to both the gut and salivary gland bocking the Plasmodium at these two stages. | ||
| + | |||
| + | |||
| + | *An other attractive approach is to block chitinase present in the mousquitoís gut. This enzyme plays an important role in modelling of the peritrofic matrix (PM, that surround the bood meal) which is an important barrier for the Plasmodium. It has been shown that inhibiting chitinase make the PM thicker and efficiently blocks the plasmodium development. Chitinase is activated by cleaving an N-terminal propeptide, and it has been demonstrated, by feeding the mosquito with the peptide, that this same propeptide, called '''prochitinase peptide''' (13 amino-acids) blocks efficiently the chinase activity and the plasmodium developement in the mosquitoís gut (sequence: [14]). | ||
| + | |||
| + | |||
| + | *The Phospholypase A2 (PLA2) inhibits oocyst formation in the mousquitoís gut by feeding or expressing it in transgenic mousquitos (See [13] and [14]). Interestingly this enzyme comes from the snake or bee venom and its anti-malaria activity does not depend on its hydrolytic activity. | ||
| + | |||
| + | |||
| + | In summary we have those potential effector molecules: | ||
| + | *SB-37 | ||
| + | *Ceropins (mainly A, B and D) | ||
| + | *Glossina attacin | ||
| + | *SM-1 | ||
| + | *Prochitinase peptide | ||
| + | *PLA2 | ||
| + | |||
| + | = References: = | ||
*[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T29-42JHDJD-9&_user=10&_coverDate=03%2F31%2F2001&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5f4b78b08a1846e241faaed33bc76cb3&searchtype=a 1. Shigeto Yoshida, ''Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes'', Molecular and Biochemical Parasitology (2001)] | *[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T29-42JHDJD-9&_user=10&_coverDate=03%2F31%2F2001&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5f4b78b08a1846e241faaed33bc76cb3&searchtype=a 1. Shigeto Yoshida, ''Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes'', Molecular and Biochemical Parasitology (2001)] | ||
| Line 70: | Line 159: | ||
*[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1951121/?tool=pubmed 3. Ajay K. Saxena, Yimin Wu, and David N. Garboczi, ''Plasmodium P25 and P28 Surface Proteins: Potential Transmission-Blocking Vaccines'', Eukaryot Cell (2007)] | *[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1951121/?tool=pubmed 3. Ajay K. Saxena, Yimin Wu, and David N. Garboczi, ''Plasmodium P25 and P28 Surface Proteins: Potential Transmission-Blocking Vaccines'', Eukaryot Cell (2007)] | ||
*[http://www.nature.com/nsmb/journal/v13/n1/full/nsmb1024.html 4. Ajay et al., The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms, natures structural & molecular biology, 2005] | *[http://www.nature.com/nsmb/journal/v13/n1/full/nsmb1024.html 4. Ajay et al., The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms, natures structural & molecular biology, 2005] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/3049204 5. Jaynes et al. 1988] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/9096383 6. Durvasula et al. 1997] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed 7. Kim et al. 2004] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/7140755 8. Hultmark et al. 1982] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/18477243 9. Wang et al., 2008] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/15894187 10. Jacobs-Lorena et al., 2005] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/11687659 11. Ghosh et al., 2001] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed 12. Bhatnagar et al., 2003] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/11809789 13. Zieler et al., 2001] | ||
| + | *[http://www.ncbi.nlm.nih.gov/pubmed/12167627 14. Moreira et al., 2002] | ||
{{EPFL_2010_bottom_wrap}} | {{EPFL_2010_bottom_wrap}} | ||
</div> | </div> | ||
Latest revision as of 20:58, 27 October 2010


Contents |
Immunotoxin
The [http://partsregistry.org/Part:BBa_K320001 immunotoxin] is composed of two main parts. The first is a single-chain antibody fragment (scFv) directed to Pbs2l, a surface membrane protein of P. berghei ookinetes. This fragment is linked to the second part, a lytic peptide, Shiva-1. Shiva-1 is a synthetic peptide with an amino acid sequence similar, in terms of length (38 amino acids, charge distribution as well as hydrophobicity, to Cecropin B which is an antibacterial peptide. The purpose of the immunotoxin is to specifically target and lyse the parasite.
Previous experiments conducted show that feeding mosquitoes with recombinant E.Coli that expressed this immunotoxin induced a significantly lower oocyst density. [ 1 ]
However E. Coli is not a natural symbiote of the mosquito. We would like to contribute to the fight against malaria by engineering Asaia in order to express the immunotoxin in the mosquito's intestinal tract, and run experiments to see if it significantly reduces the number of oocysts.
The sequence of the immunotoxin was taken from our reference paper [ 1 ], and the codons were optimized for E.Coli. The codon optimized sequence can be found here.
The "P-proteins"
P25 and P28 are a class of important proteins expressed on the membrane of different type of Plasmodium; we call this ensemble of evolutionary conserved proteins the P-proteins. They are mainly expressed on the mosquito-stage parasite (ookinete). The ookinete has been intensively studied by scientists, looking for an ideal transmission-blocking vaccine target.
Why the P-proteins?
Plasmodium proteins [http://partsregistry.org/Part:BBa_K320007 P25] and [http://partsregistry.org/Part:BBa_K320009 P28] are important for the protection of P. falciparum. In fact, they are very abundant on the ookinete surface and this coating prevents the action of hard proteolytic environment from the gut and reduces the exposition of the ookinete to it. Other functions of the P-proteins are involved with gametes fusion as well as ookinete entry into midgut epithelial cells. The process of penetration through the epithelium is essential for the ookinete maturation and propagation and experiments have shown that double-knockout of these proteins significantly reduces the migration of parasites across the midgut. An interesting observation is that parasites lacking either P25 or P28 are transmitted efficiently by mosquitoes. It is rather the double knockout of these proteins that compromises greatly the parasites propagation by the mosquito. In fact, it has been shown that the binding of specific antibodies to these surface proteins was fatal to more than 99% of the parasites by inhibiting their interactions [ 2 ]. This indicates that these two proteins have partially redundant functions. Experiments have shown that even when the ookinete could traverse the midgut epithelium these proteins are important for further development of the ookinete into oocysts [ 3 ].
The team decided to design Asaia to express a soluble form of these two P-proteins. It may seem contra-intuitive to provide more of these proteins (by expression in Asaia) that are essential for survival, migration, and development of the parasite. However, our hypothesis is based on the fact that these proteins over-expressed in a soluble form would compete with the parasite surface protein and prevent the interaction necessary to its transmission.
Because the functions of P25 and P28 are redundant, both proteins need to be inhibited or outcompeted to efficiently block the malaria transmission.
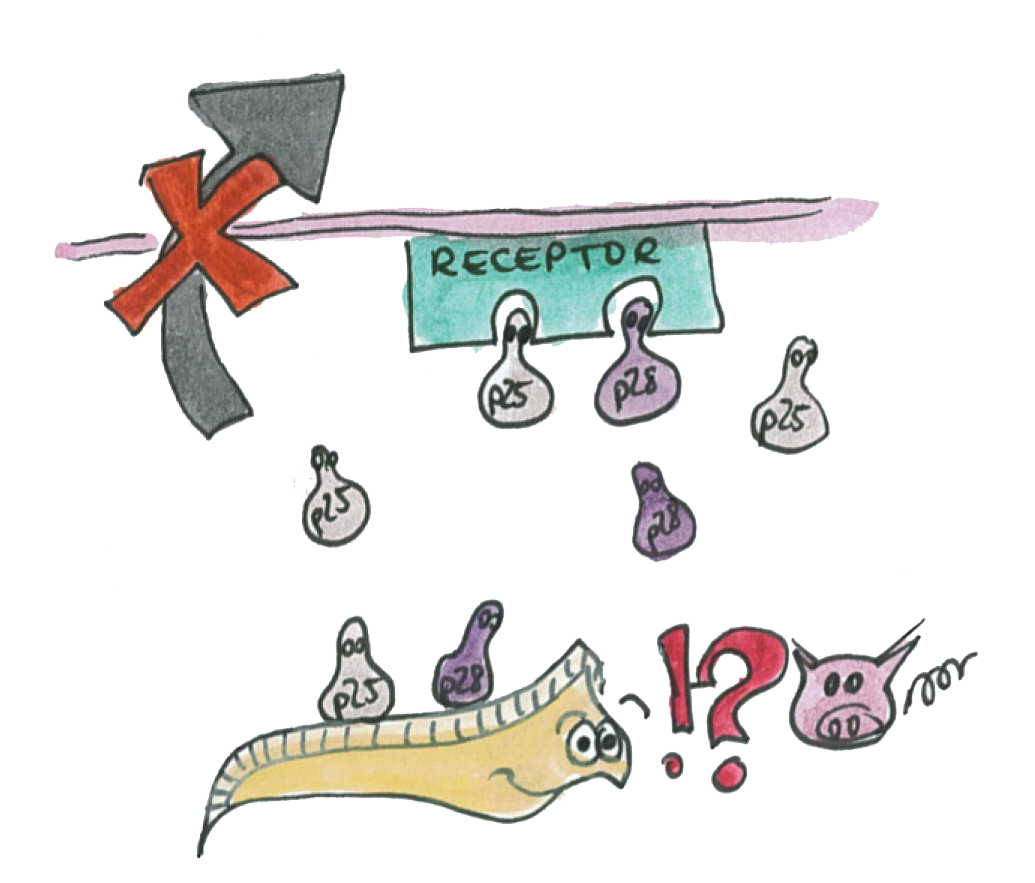
As illustrated on the picture, the P-proteins mediate the transmission of the parasite through the epithelium.
We know that a double knockout of these proteins make it impossible for the parasite to traverse the epithelium.
We also know that antibodies specific to the P-proteins also inhibit the parasite transmission.
However, if we are able to express the P-proteins by Asia in the mosquito's gut the P-protein interactions will be disrupted and the parasite will not we able to cross the epithelium.
Structural information about P-proteins
The two P-proteins were conserved throughout evolution. They have a C-terminal glycosylphosphatidylinositol that anchors the proteins on the plasmodium membrane so that it is accessible from the extracellular side.
Sizes of the genes: The two genes does not contain introns so that we can directly perform a PCR on the genomic DNA of the plasmodium. The length of P25 and P28 genes are 654 and 657 bp respectively.
An interesting feature of the X-ray crystal structure of the Pvs25 : the four EGF-like domains form a compact triangular prism. These prisms are arranged on layers of sheets in the crystal. P. falciparum P25 (Pfs25) likely assume a similar structure (see Figure 1 and crystal structure). These structures may play a role of protection of the parasite, cysteins play an important role to maintain this structure by making 11 and 10 disulfide bonds in the P25 and P28 protein respectively.

In order to find the protein, we attached a His tag to the P-proteins when we designed the primers to extract the proteins from the Plasmodium gDNA. We attached it to the C-terminus. The reason for that is that the C-terminus is involved in the attachment of the protein to the plasmodium outer membrane and thus should not e involved on the P-protein – P-protein and P-protein – Receptor interactions. This idea is supported by experiments with transmission-blocking antibodies. Indeed wee can see on the figure that the epitop of the transmission-blocking antibody is far away from the C-terminus. So we think that the addition of the His-tag does not compromise the functionalities of the proteins.

Here is the X-ray crystal structure of P25: [http://www.pdb.org/pdb/explore/jmol.do?structureId=1Z1Y&bionumber=1 Crystal structure]
Candidate effector molecules to block malaria propagation
At the beginning of our project, we have conducted further research in order to find all possible ways to block the transmission of malaria. Here you can find some of the most interesting ideas:
- A lytic peptide called SB-37 similar (in term of length and amino acids property) to Shiva-1 (the lytic peptide of our ordered immunotoxin) was used to kill P. falciparum in vitro [5]. SB-37 is a slightly modified version of a Cecropin, the Cecropin B [5], the sequence is in the paper [5].
- Cecropins are peptides derived from the insect immune defence (a giant moth). Cecropin A was expressed in a simbiont of the bug Rhodnius prolixus in order to kill T. cruzi, a parasite that cause the Chagas disease [6]. More recently, It was also expressed in the mosquitoís gut (transgenic mosquitoes) to prevent the propagation of the malaria [7] (See [http://www.anaspec.com/products/product.asp?id=30699 Sequence]). Because of its hight homology the cecropins A and B, cecropin D could also be used (sequence: [7]). There exists 6 cecropins : cecropin A, Ö , F.
- The Glossina attacin is an antimicrobial peptide effective on both gram-negative bacteria and protozoa (Plasmodium is a protozoa!). It was expressed in a symbiont of the tsetse fly in order to kill the pasasite T.brucei; We should determine if it acts on Asaia, attacins kill E.Coli but [http://www.copewithcytokines.org/cope.cgi?key=Attacins do not act on many other gram-positive and gram-negative bacteria]. Sequence: [9].
Other effectors molecules against Plasmodium are cited by Jacobs-Lorena and al. [10]:
- The salivary gland/midgut peptide (SM1), it is a dodecapeptide (sequence: [11]), It was found by testing a number of radom peptides, and it binds to both the gut and salivary gland bocking the Plasmodium at these two stages.
- An other attractive approach is to block chitinase present in the mousquitoís gut. This enzyme plays an important role in modelling of the peritrofic matrix (PM, that surround the bood meal) which is an important barrier for the Plasmodium. It has been shown that inhibiting chitinase make the PM thicker and efficiently blocks the plasmodium development. Chitinase is activated by cleaving an N-terminal propeptide, and it has been demonstrated, by feeding the mosquito with the peptide, that this same propeptide, called prochitinase peptide (13 amino-acids) blocks efficiently the chinase activity and the plasmodium developement in the mosquitoís gut (sequence: [14]).
- The Phospholypase A2 (PLA2) inhibits oocyst formation in the mousquitoís gut by feeding or expressing it in transgenic mousquitos (See [13] and [14]). Interestingly this enzyme comes from the snake or bee venom and its anti-malaria activity does not depend on its hydrolytic activity.
In summary we have those potential effector molecules:
- SB-37
- Ceropins (mainly A, B and D)
- Glossina attacin
- SM-1
- Prochitinase peptide
- PLA2
References:
- [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T29-42JHDJD-9&_user=10&_coverDate=03%2F31%2F2001&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5f4b78b08a1846e241faaed33bc76cb3&searchtype=a 1. Shigeto Yoshida, Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes, Molecular and Biochemical Parasitology (2001)]
- [http://www.nature.com/emboj/journal/v20/n15/full/7593895a.html 2. Ana M. Tomas, Gabriele Margos, Robert E. Sinden, P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions, The EMBO Journal (2001)]
- [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1951121/?tool=pubmed 3. Ajay K. Saxena, Yimin Wu, and David N. Garboczi, Plasmodium P25 and P28 Surface Proteins: Potential Transmission-Blocking Vaccines, Eukaryot Cell (2007)]
- [http://www.nature.com/nsmb/journal/v13/n1/full/nsmb1024.html 4. Ajay et al., The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms, natures structural & molecular biology, 2005]
- [http://www.ncbi.nlm.nih.gov/pubmed/3049204 5. Jaynes et al. 1988]
- [http://www.ncbi.nlm.nih.gov/pubmed/9096383 6. Durvasula et al. 1997]
- [http://www.ncbi.nlm.nih.gov/pubmed 7. Kim et al. 2004]
- [http://www.ncbi.nlm.nih.gov/pubmed/7140755 8. Hultmark et al. 1982]
- [http://www.ncbi.nlm.nih.gov/pubmed/18477243 9. Wang et al., 2008]
- [http://www.ncbi.nlm.nih.gov/pubmed/15894187 10. Jacobs-Lorena et al., 2005]
- [http://www.ncbi.nlm.nih.gov/pubmed/11687659 11. Ghosh et al., 2001]
- [http://www.ncbi.nlm.nih.gov/pubmed 12. Bhatnagar et al., 2003]
- [http://www.ncbi.nlm.nih.gov/pubmed/11809789 13. Zieler et al., 2001]
- [http://www.ncbi.nlm.nih.gov/pubmed/12167627 14. Moreira et al., 2002]

 "
"