Team:Tokyo Tech/Project/Artificial Cooperation System/lux act rep/Pluxrep/assay2
From 2010.igem.org
(New page: {{Tokyo_Tech_Template2}} <div id="super_main_wrapper"> <div id="tf_menu"> menu </div> <!-- tf_menu --> <div id="tf_SubWrapper"> <!--ここから書き始めて下さい--> <!--...) |
(→Materials & Methods) |
||
| (One intermediate revision not shown) | |||
| Line 9: | Line 9: | ||
<!--ここから書き始めて下さい--> | <!--ここから書き始めて下さい--> | ||
| + | =luxR repression promoter (BBa_K395008) assay= | ||
| + | ===Abstract=== | ||
| + | We wanted to characterize the strength of this promoter which we designed. We measured fluorescence by flow cytometry 3 hour after addition of 100nM 3OC6HSL to confirm K395008, which is a promoter repressed by LuxR/3OC6HSL complex. | ||
| + | ===Introduction=== | ||
| + | Even subtle changes in promoter may have distinct effects on the expression of gene. We designed a new promoter, K395008, which is repressed by LuxR/3OC6HSL complex by changing one base of the existing BioBrick parts (BBa_R0061). We wanted to characterize this luxR repression promoter. Also, we wanted to confirm that this promoter is also repressed by LuxR/3OC6HSL complex but has different strength from the existing BioBrick part. | ||
| + | ===Results=== | ||
| + | The result is shown in fig.○. | ||
| + | The expression of GFP with 3OC6HSL dropped to 1/3 comparing with the expression without 3OC6HSL. | ||
| + | ===Conclusion=== | ||
| + | We confirmed that 3OC6HSL repressed luxR repression promoter, K395008, as expected. | ||
| + | ===Materials & Methods=== | ||
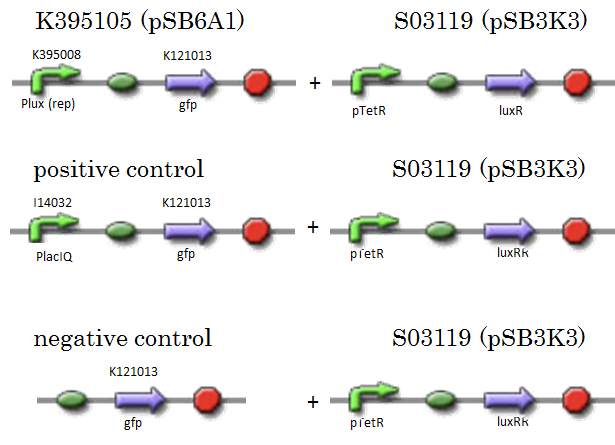
| + | We constructed K395105 combining K395008 and K121013. K121013 is a promoter-less gfp reporter (rbs-gfp-ter-ter) and this backbone is pSB6A1. Promoter of S03119 is PtetR, which is repressed by tetR. In this experiment, we don’t use TetR, so, S03119 functions a LuxR constitutive generator. The backbone of S03119 is pSB1A2, which is a high copy plasmid, so we changed the backbone from pSB1A2 to pSB3K3. | ||
| + | We used a fusion of PlacI<sup>q</sup> (I14032) to gfp (K121013) as a positive control and used promoterless gfp (K121013) as a negative control. | ||
| + | |||
| + | [[IMAGE:Tokyotech_K395008assay_construction.png|400px]] | ||
| + | |||
| + | *samples | ||
| + | #[R0061weak - GFP](BBa_K395105) on pSB6A1 + [PtetR – LuxR] on pSB3K3 | ||
| + | #positive control: [PlacI<sup>q</sup>(constitutive promoter) - GFP] on pSB6A1+ [PtetR – LuxR]) on pSB3K3 | ||
| + | #negative control: [promoterless - GFP] on pSB6A1+ [PtetR – LuxR] on pSB3K3 | ||
| + | *Strain | ||
| + | DH5&alpha | ||
| + | *protocol | ||
| + | #Prepare overnight culture. | ||
| + | #Take 30 ul of the overnight culture into LB + antibiotics (Amp + Kan). (→fresh culture) | ||
| + | #Incubate the fresh culture until the observed O.D. reaches around 0.60. | ||
| + | #Each sample was divided into 2. Prepare and add 3OC6HSL mixture to one, and add DMSO mixture to the other. The final concentration of 3OC6HSL is 100nM. | ||
| + | #Induction for 3 hours at 37°C. | ||
| + | #Fluorometer (FLA5200) and flow cytometry measurements for GFP expression. | ||
| + | |||
| + | ===Reference=== | ||
| + | #KRISTI A. EGLAND & E. P. GREENBERG Conversion of the Vibrio fischeri Transcriptional Activator, LuxR, to a Repressor JOURNAL OF BACTERIOLOGY, Feb. 2000, p. 805–811 | ||
Latest revision as of 14:39, 26 October 2010
Contents |
luxR repression promoter (BBa_K395008) assay
Abstract
We wanted to characterize the strength of this promoter which we designed. We measured fluorescence by flow cytometry 3 hour after addition of 100nM 3OC6HSL to confirm K395008, which is a promoter repressed by LuxR/3OC6HSL complex.
Introduction
Even subtle changes in promoter may have distinct effects on the expression of gene. We designed a new promoter, K395008, which is repressed by LuxR/3OC6HSL complex by changing one base of the existing BioBrick parts (BBa_R0061). We wanted to characterize this luxR repression promoter. Also, we wanted to confirm that this promoter is also repressed by LuxR/3OC6HSL complex but has different strength from the existing BioBrick part.
Results
The result is shown in fig.○. The expression of GFP with 3OC6HSL dropped to 1/3 comparing with the expression without 3OC6HSL.
Conclusion
We confirmed that 3OC6HSL repressed luxR repression promoter, K395008, as expected.
Materials & Methods
We constructed K395105 combining K395008 and K121013. K121013 is a promoter-less gfp reporter (rbs-gfp-ter-ter) and this backbone is pSB6A1. Promoter of S03119 is PtetR, which is repressed by tetR. In this experiment, we don’t use TetR, so, S03119 functions a LuxR constitutive generator. The backbone of S03119 is pSB1A2, which is a high copy plasmid, so we changed the backbone from pSB1A2 to pSB3K3. We used a fusion of PlacIq (I14032) to gfp (K121013) as a positive control and used promoterless gfp (K121013) as a negative control.
- samples
- [R0061weak - GFP](BBa_K395105) on pSB6A1 + [PtetR – LuxR] on pSB3K3
- positive control: [PlacIq(constitutive promoter) - GFP] on pSB6A1+ [PtetR – LuxR]) on pSB3K3
- negative control: [promoterless - GFP] on pSB6A1+ [PtetR – LuxR] on pSB3K3
- Strain
DH5&alpha
- protocol
- Prepare overnight culture.
- Take 30 ul of the overnight culture into LB + antibiotics (Amp + Kan). (→fresh culture)
- Incubate the fresh culture until the observed O.D. reaches around 0.60.
- Each sample was divided into 2. Prepare and add 3OC6HSL mixture to one, and add DMSO mixture to the other. The final concentration of 3OC6HSL is 100nM.
- Induction for 3 hours at 37°C.
- Fluorometer (FLA5200) and flow cytometry measurements for GFP expression.
Reference
- KRISTI A. EGLAND & E. P. GREENBERG Conversion of the Vibrio fischeri Transcriptional Activator, LuxR, to a Repressor JOURNAL OF BACTERIOLOGY, Feb. 2000, p. 805–811
 "
"