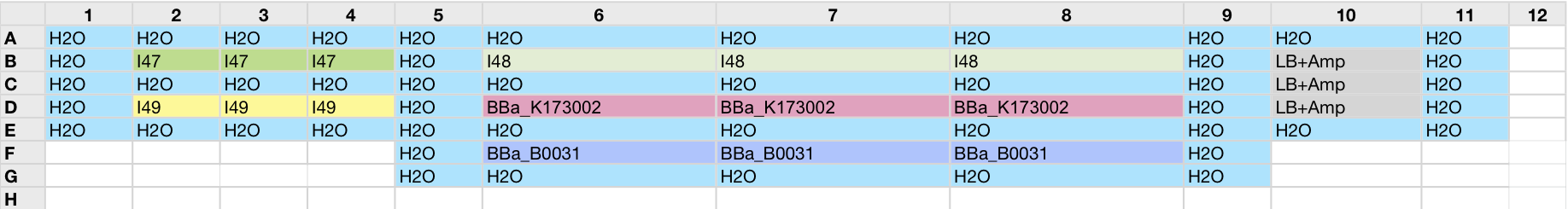
Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test27settembre
From 2010.igem.org
m (→Methods) |
|||
| (9 intermediate revisions not shown) | |||
| Line 19: | Line 19: | ||
==EXPERIMENT DESCRIPTION== | ==EXPERIMENT DESCRIPTION== | ||
===Motivation=== | ===Motivation=== | ||
| - | This experiment was performed to check bacterial growth and GFP | + | This experiment was performed to check bacterial growth and GFP synthesis rate of the following constructs in order to verify right protein folding. |
===Methods=== | ===Methods=== | ||
| Line 28: | Line 28: | ||
*<partinfo>BBa_K173000</partinfo> (positive control) | *<partinfo>BBa_K173000</partinfo> (positive control) | ||
*<partinfo>BBa_B0031</partinfo> (negative control) | *<partinfo>BBa_B0031</partinfo> (negative control) | ||
| - | + | Cultures were grown ON at 37°C, 220 rpm. | |
| - | The following day cultures were diluted 1:100 and let grow again for about five hours at | + | The following day cultures were diluted 1:100 and let grow again for about five hours at 37°C, 220 rpm. |
| - | + | The optical density (O.D.) of each cell culture was than measured with TECAN Infinte F200. Samples were diluted in order to obtain the same O.D. equal to 0,02. | |
| - | + | Then we performed a 21-hours' experiment with measurements of absorbance and green fluorescence every five minutes using TECAN Infinite F200; cultures were shaken for 15 seconds every five minutes. Each value shown below is the mean of three measurements, from GFP data that of a non-fluorescent culture (negative control) was subtracted. | |
==RESULTS== | ==RESULTS== | ||
| Line 42: | Line 42: | ||
<table border="1"> | <table border="1"> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <th>Culture</th><th>Doubling time [min.]</th> | + | <th>Culture</th><th>Doubling time [min.] ± std error</th> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td><partinfo>BBa_K173000</partinfo></td><td> | + | <td><partinfo>BBa_K173000</partinfo></td><td>76.3336 ± 1.4362</td> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td>I47</td><td> | + | <td>I47</td><td>73.6685 ± 1.6245</td> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td>I48</td><td> | + | <td>I48</td><td>74.8806 ± 2.7699</td> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td>I49</td><td> | + | <td>I49</td><td>75.9433 ± 3.6808</td> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td><partinfo>BBa_B0031</partinfo></td><td> | + | <td><partinfo>BBa_B0031</partinfo></td><td>70.8421 ± 2.2181</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 64: | Line 64: | ||
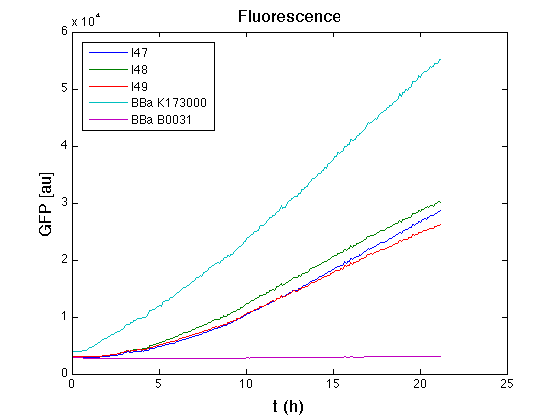
[[Image:UNIPV_Pavia_GFP27settembre.png|thumb|500px |center|Raw GFP curve]] | [[Image:UNIPV_Pavia_GFP27settembre.png|thumb|500px |center|Raw GFP curve]] | ||
| - | [[Image:UNIPV_Pavia_SScellM27settembre.png|thumb|500px |center|Mean of (dGFP/dt)/O.D.600 (under the hypothesis that half-life | + | [[Image:UNIPV_Pavia_SScellM27settembre.png|thumb|500px |center|Mean of (dGFP/dt)/O.D.600 (under the hypothesis that GFP half-life in fusion contructs is similar to the original one - <partinfo>BBa_E0040</partinfo>)]] |
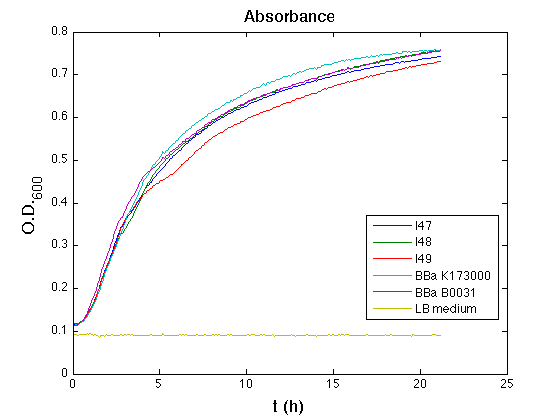
| - | All cell cultures showed a similar growth curve | + | All cell cultures showed a similar growth curve; doubling time was computed as described [[Team:UNIPV-Pavia/Parts/Characterization#Doubling_time_evaluation|here]] in order to have informations about the burden due to synthesis of such fusion proteins. It's possible to see that all doubling time are very similar; it's possible to assert that the expression of these BioBrick parts doesn't cause abnormal stress to cells. |
| - | + | From GFP curve it's possible to appreciate that in I47, I48, I49 GFP accumulation it's very similar and it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. This result that the green fluorescent protein assembled downstream of the genetic circuit is correctly folded. | |
| - | + | The mean protein synthesis rate was also computed over the growth exponential phase, showing again an appreciable GFP production rate that is about a half of the positive control. | |
<!-- table previous next test --> | <!-- table previous next test --> | ||
Latest revision as of 22:24, 27 October 2010
|
|
||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||
 "
"