Team:ETHZ Basel/Modeling/Light Switch
From 2010.igem.org
(→Background) |
(→Facing the Combinatorial Explosion) |
||
| (48 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{ETHZ_Basel10_Modeling}} | {{ETHZ_Basel10_Modeling}} | ||
| - | = | + | = The light switch = |
| - | [[ | + | We created two models representing the different biological approaches on how to control the tumbling frequency of the E. lemming, |
| + | # a model representing the [[Team:ETHZ_Basel/Modeling/Light_Switch#Modeling of the PhyB/PIF3 light switch|PhyB/PIF3 based localization]] of proteins of the chemotaxis pathway, | ||
| + | # a model representing the [[Team:ETHZ_Basel/Modeling/Light_Switch#Archeal light receptor|Archeal light receptor]]. | ||
| - | == Background == | + | == Modeling of the PhyB/PIF3 light switch == |
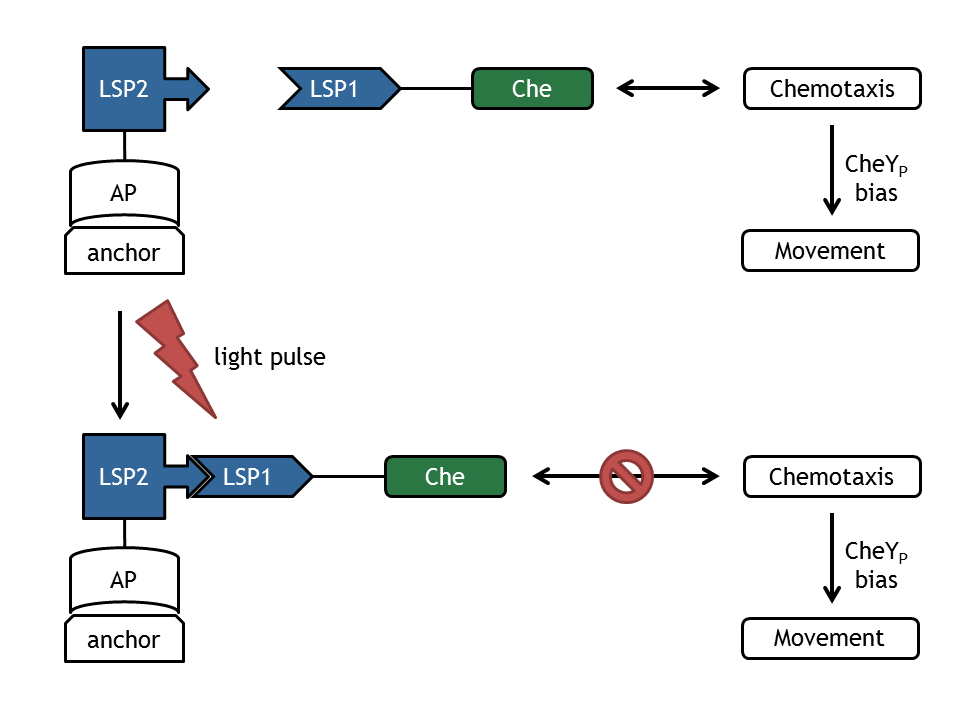
| - | In our biological setup, the relocation of one of the chemotaxis pathway proteins (either CheR, CheB, CheY or CheZ) is achieved by fusing them to a light-sensitive protein LSP1 (either to PhyB or to PIF3), which dimerizes by red light induction with the corresponding LSP2 (PIF3 or PhyB), fused to a spatially dislocated anchor. Since we decided to implement two different models of the chemotaxis pathway (see | + | [[Image:ETHZ_Basel_molecular_comb.png|thumb|400px|'''Figure 1: Schematic overview of the devices and change upon light pulse induction.''' LSP refers to light switch protein, AP to anchor protein and Che to the attacked protein of the chemotaxis pathway.]] |
| + | |||
| + | === Background === | ||
| + | In our biological setup, the relocation of one of the chemotaxis pathway proteins (either CheR, CheB, CheY or CheZ) is achieved by fusing them to a light-sensitive protein LSP1 (either to PhyB or to PIF3), which dimerizes by red light induction with the corresponding LSP2 (PIF3 or PhyB), fused to a spatially dislocated anchor. Since we decided to implement two different models of the chemotaxis pathway (see section [[Team:ETHZ_Basel/Modeling/Chemotaxis|Chemotaxis Pathway]]), modeling all setups implemented in the wet-lab would have resulted in 16 different models: | ||
|{CheR, CheB, CheY, CheZ} × {PhyB, PIF3} × {Model 1, Model 2}|=16. | |{CheR, CheB, CheY, CheZ} × {PhyB, PIF3} × {Model 1, Model 2}|=16. | ||
| - | Inspired by the modular approach used | + | Inspired by the modular approach used for the [[Team:ETHZ_Basel/Biology/Cloning|Cloning Strategy]] in the wet-lab, we decided to decrease the combinatorial explosion by also applying a novel modular approach. Not only did this approach reduce the amount of models by a factor of four, but it also allowed to widely separate the differential equations of the chemotaxis pathway from those of the light-induced localization system by simultaneously decreasing redundancies and thus decreasing the danger of slip of the pens. The underlying mathematical model for the light-induced relocation was completely developed by us (for a short discussion of the recently by Sorokina et al. published light-induced relocation system and why we did not use it, see [[Team:ETHZ_Basel/Modeling/Sorokina|here]]) as well as - to our knowledge - the approach to couple this model to models of the chemotaxis pathway. |
| - | == | + | === Assumptions === |
| - | + | The following assumptions have been made according to the [[Team:ETHZ_Basel/Biology/Molecular_Mechanism| molecular mechanism]] to link the light switch and chemotaxis models: | |
| - | + | ||
| - | + | * Upon red light pulse induction, the two light-sensitive protein (LSP1 and LSP2) domains can hetero-dimerize. | |
| + | * The TetR-LSP2 can bind to the operator (tetO) on the DNA. | ||
| + | * Only after both reactions took place, we assume the Che protein to be spatially dislocated. This means | ||
| + | ** CheR-LSP1 is not able to methylate the MCPs anymore, | ||
| + | ** CheY-LSP1 can't be phosphorylated and interact with the motor anymore; nevertheless, it still can be dephosphorylated. | ||
| + | ** CheB-LSP1 is not able to demethylate the MCPs and can't be phosphorylated anymore, but still can be dephosphorylated, | ||
| + | ** CheZ-LSP1 can't dephosphorylate CheY anymore (This assumption is very unsteady, since CheY is not strictly located). | ||
| + | * If only one of the reactions took place, we assume that the localization and activity of the fusion proteins are the same as for wild-type cells. | ||
| - | + | All of these assumptions will lead to a decrease of tumbling / directed movement ratio upon red light induction and an increase of corresponding far-red light induction. | |
| - | + | ||
| - | + | === Facing the Combinatorial Explosion === | |
| + | The main problem in separating <i>in silico</i> the chemotaxis pathway from the light-induced relocation system is the non-existence of a hierarchical relationship between the two sub-models: The properties of the chemotaxis pathway are clearly influenced if the ''active'' concentration of one of its key species drops, such that a natural strategy that stands out is giving the amount of localized proteins, obtained by the localization model, as an input to the chemotaxis model. However, also the concentration of the localized species of the localization model change depending on the reactions in the chemotaxis model, since several of the Che proteins are modeled as two or more molecular species. For example, the CheY protein is modeled as two species, one representing the phosphorylated and one the non-phosphorylated molecular concentration. Thus, the two models cannot be represented as e.g. a hierarchical block structure. This fact makes a modular approach significant more complicated. | ||
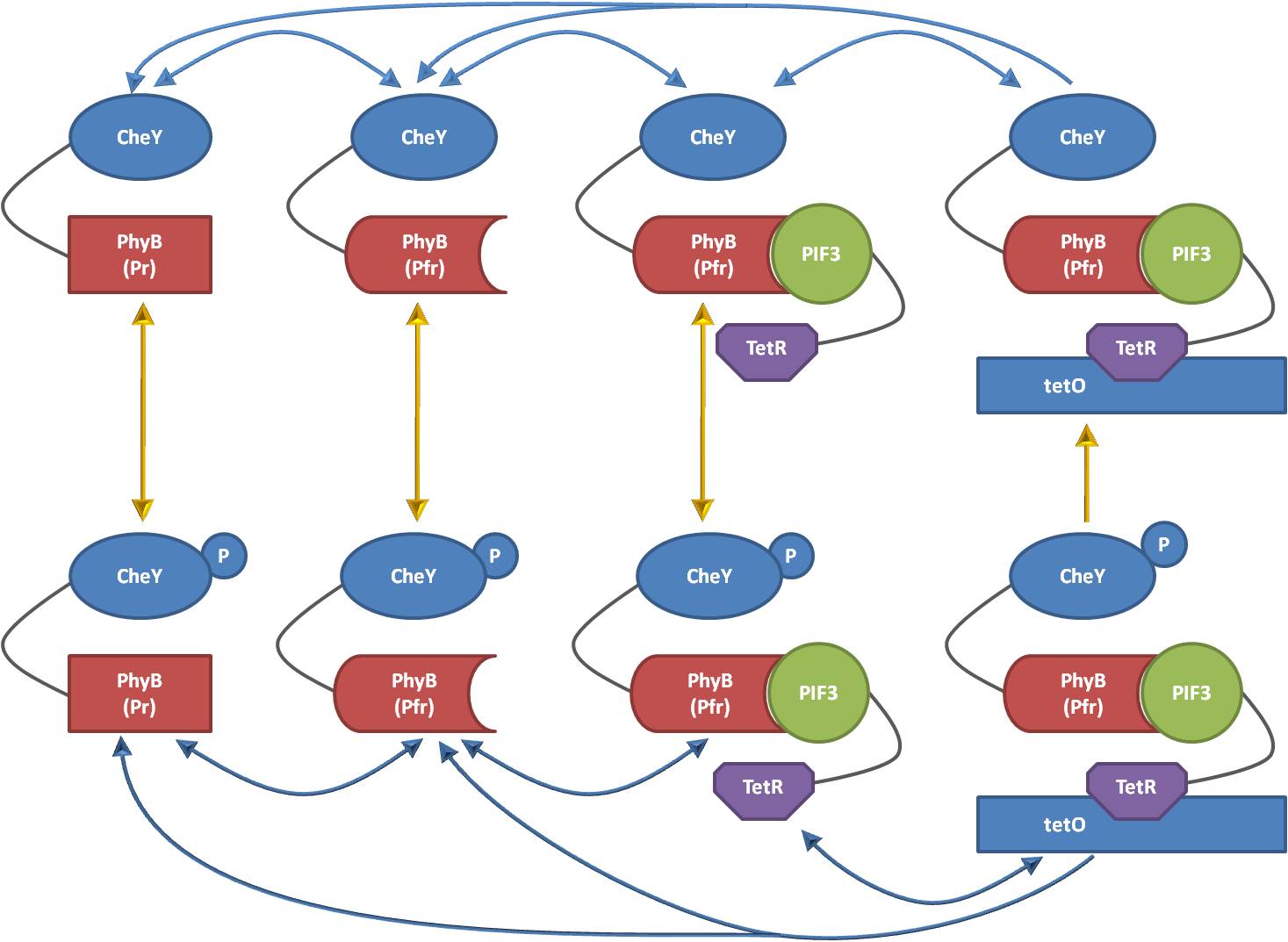
| - | + | Our approach to modularize the model is based on an observation of the reaction directions which can take place in the overall model. For example, Figure 2 shows all eight species which have to be implemented to adequately describe the localization and phosphorylation states of CheY when fused to PhyB (the reactions taking place without CheY-PhyB, as for example the binding and unbinding of the PIF3-TetR monomer to tetO, are not depicted for simplicity). As can be seen, the reactions of the chemotaxis pathway and the reactions of the light model have different directions. The reactions of the light model are horizontal and independent of the phosphorylation level of CheY. On the other hand, the reactions of the chemotaxis model are vertically. However, one of the reactions of the chemotaxis model is not independent of the states mainly influenced by the light model: When CheY is localized at the DNA, the receptor complex cannot transfer a phosphor to it anymore. The respective species diagrams for the other three species (CheZ, CheB, CheR) as well as for the other light model (the Che protein is fused to PIF3 instead of PhyB) look similar, although the number of rows and columns can change. | |
| + | [[Image:ETHZ_Basel_chemotaxis_CheYSpecies.jpg|thumb|center|550px|'''Figure 2: Different species representing different localization and phosphorylation states of CheY.]] | ||
| - | + | We used this regularity to modularize the overall model: A variable table of states representing the concentration of all Che proteins in their different phosphorylation and localization modes is shared between the light model and the chemotaxis model. The number of rows of this table is dynamically determined by the chemotaxis model and the number of columns is determined by the light model, depending on the choice of which Che protein is fused to which light sensitive protein. The light model may only change the distribution of the species in each row, but may not change their sum (conservation relation). The chemotaxis model on the other hand may only change the distribution of the species concentration in each column. Although the tables may have different sizes, always the last column is representing the localized proteins. The chemotaxis model thus only applies a subset of reactions to this row, as mentioned in the assumptions. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | All other species of both models are not shared between the two models, which also in any other way as mentioned above act independently. Using this approach we succeeded in uncoupling the model of the PhyB/PIF3 system from the model of the chemotaxis pathway, reducing the amount of redundancy and possible error sources significantly. | |
| - | == | + | == Archeal light receptor == |
| - | + | Another modeling technique we employed in implementing the E. lemming was to fuse an archaeal photoreceptor to the'' E. coli'' chemotactic transducer, i.e. we replaced the usual receptor of E. coli that binds & is activated by chemo-attractant, with a receptor that is activated by blue-green light as demonstrated by Jung et al. (2001) [[Team:ETHZ Basel/Modeling/Light_Switch#References|[1]]]. | |
| - | [ | + | For the description of the molecular processes we could use our already [[Team:ETHZ_Basel/Modeling/Chemotaxis|implemented chemotaxis models]] from which we picked the chemotaxis model suggested by [[Team:ETHZ_Basel/Modeling/Chemotaxis|Mello & Tu (2003)]]. We had to replace the interaction between the chemo-attractant and the receptor with a interaction between light and the receptor which gives rise to the same set of events down stream of the chemotaxis pathway. |
| - | [ | + | The strategy to model this interaction was to define the dynamic receptor occupancy as a dynamic function of the light input. The receptor occupancy reflects the fraction of receptors activated by the stimulus (chemo-attractant or light). |
| + | |||
| + | With the help of this implementation, we were able to display the internal CheYp concentration, to estimate the photoreceptor occupancy and to calculate the bias as shown in the [[Team:ETHZ_Basel/Achievements/E_lemming|E. lemming section]]. | ||
| + | |||
| + | == Download == | ||
| + | Both light switch models are included within the [[Team:ETHZ_Basel/Achievements/Matlab_Toolbox|Matlab Toolbox]] and can be downloaded there. | ||
| + | |||
| + | == References == | ||
| + | [1] [http://jb.asm.org/cgi/reprint/183/21/6365?maxtoshow=&hits=10&RESULTFORMAT=1&andorexacttitle=and&andorexacttitleabs=and&fulltext=An+archaeal+photosignal-transducing+module+mediates+phototaxis+in+%27%27Escherichia+&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT: Jung et al: An Archaeal Photosignal-Transducing Module Mediates Phototaxis in Escherichia coli] | ||
Latest revision as of 03:55, 28 October 2010
The light switch
We created two models representing the different biological approaches on how to control the tumbling frequency of the E. lemming,
- a model representing the PhyB/PIF3 based localization of proteins of the chemotaxis pathway,
- a model representing the Archeal light receptor.
Modeling of the PhyB/PIF3 light switch
Background
In our biological setup, the relocation of one of the chemotaxis pathway proteins (either CheR, CheB, CheY or CheZ) is achieved by fusing them to a light-sensitive protein LSP1 (either to PhyB or to PIF3), which dimerizes by red light induction with the corresponding LSP2 (PIF3 or PhyB), fused to a spatially dislocated anchor. Since we decided to implement two different models of the chemotaxis pathway (see section Chemotaxis Pathway), modeling all setups implemented in the wet-lab would have resulted in 16 different models:
|{CheR, CheB, CheY, CheZ} × {PhyB, PIF3} × {Model 1, Model 2}|=16.
Inspired by the modular approach used for the Cloning Strategy in the wet-lab, we decided to decrease the combinatorial explosion by also applying a novel modular approach. Not only did this approach reduce the amount of models by a factor of four, but it also allowed to widely separate the differential equations of the chemotaxis pathway from those of the light-induced localization system by simultaneously decreasing redundancies and thus decreasing the danger of slip of the pens. The underlying mathematical model for the light-induced relocation was completely developed by us (for a short discussion of the recently by Sorokina et al. published light-induced relocation system and why we did not use it, see here) as well as - to our knowledge - the approach to couple this model to models of the chemotaxis pathway.
Assumptions
The following assumptions have been made according to the molecular mechanism to link the light switch and chemotaxis models:
- Upon red light pulse induction, the two light-sensitive protein (LSP1 and LSP2) domains can hetero-dimerize.
- The TetR-LSP2 can bind to the operator (tetO) on the DNA.
- Only after both reactions took place, we assume the Che protein to be spatially dislocated. This means
- CheR-LSP1 is not able to methylate the MCPs anymore,
- CheY-LSP1 can't be phosphorylated and interact with the motor anymore; nevertheless, it still can be dephosphorylated.
- CheB-LSP1 is not able to demethylate the MCPs and can't be phosphorylated anymore, but still can be dephosphorylated,
- CheZ-LSP1 can't dephosphorylate CheY anymore (This assumption is very unsteady, since CheY is not strictly located).
- If only one of the reactions took place, we assume that the localization and activity of the fusion proteins are the same as for wild-type cells.
All of these assumptions will lead to a decrease of tumbling / directed movement ratio upon red light induction and an increase of corresponding far-red light induction.
Facing the Combinatorial Explosion
The main problem in separating in silico the chemotaxis pathway from the light-induced relocation system is the non-existence of a hierarchical relationship between the two sub-models: The properties of the chemotaxis pathway are clearly influenced if the active concentration of one of its key species drops, such that a natural strategy that stands out is giving the amount of localized proteins, obtained by the localization model, as an input to the chemotaxis model. However, also the concentration of the localized species of the localization model change depending on the reactions in the chemotaxis model, since several of the Che proteins are modeled as two or more molecular species. For example, the CheY protein is modeled as two species, one representing the phosphorylated and one the non-phosphorylated molecular concentration. Thus, the two models cannot be represented as e.g. a hierarchical block structure. This fact makes a modular approach significant more complicated.
Our approach to modularize the model is based on an observation of the reaction directions which can take place in the overall model. For example, Figure 2 shows all eight species which have to be implemented to adequately describe the localization and phosphorylation states of CheY when fused to PhyB (the reactions taking place without CheY-PhyB, as for example the binding and unbinding of the PIF3-TetR monomer to tetO, are not depicted for simplicity). As can be seen, the reactions of the chemotaxis pathway and the reactions of the light model have different directions. The reactions of the light model are horizontal and independent of the phosphorylation level of CheY. On the other hand, the reactions of the chemotaxis model are vertically. However, one of the reactions of the chemotaxis model is not independent of the states mainly influenced by the light model: When CheY is localized at the DNA, the receptor complex cannot transfer a phosphor to it anymore. The respective species diagrams for the other three species (CheZ, CheB, CheR) as well as for the other light model (the Che protein is fused to PIF3 instead of PhyB) look similar, although the number of rows and columns can change.
We used this regularity to modularize the overall model: A variable table of states representing the concentration of all Che proteins in their different phosphorylation and localization modes is shared between the light model and the chemotaxis model. The number of rows of this table is dynamically determined by the chemotaxis model and the number of columns is determined by the light model, depending on the choice of which Che protein is fused to which light sensitive protein. The light model may only change the distribution of the species in each row, but may not change their sum (conservation relation). The chemotaxis model on the other hand may only change the distribution of the species concentration in each column. Although the tables may have different sizes, always the last column is representing the localized proteins. The chemotaxis model thus only applies a subset of reactions to this row, as mentioned in the assumptions.
All other species of both models are not shared between the two models, which also in any other way as mentioned above act independently. Using this approach we succeeded in uncoupling the model of the PhyB/PIF3 system from the model of the chemotaxis pathway, reducing the amount of redundancy and possible error sources significantly.
Archeal light receptor
Another modeling technique we employed in implementing the E. lemming was to fuse an archaeal photoreceptor to the E. coli chemotactic transducer, i.e. we replaced the usual receptor of E. coli that binds & is activated by chemo-attractant, with a receptor that is activated by blue-green light as demonstrated by Jung et al. (2001) [1].
For the description of the molecular processes we could use our already implemented chemotaxis models from which we picked the chemotaxis model suggested by Mello & Tu (2003). We had to replace the interaction between the chemo-attractant and the receptor with a interaction between light and the receptor which gives rise to the same set of events down stream of the chemotaxis pathway.
The strategy to model this interaction was to define the dynamic receptor occupancy as a dynamic function of the light input. The receptor occupancy reflects the fraction of receptors activated by the stimulus (chemo-attractant or light).
With the help of this implementation, we were able to display the internal CheYp concentration, to estimate the photoreceptor occupancy and to calculate the bias as shown in the E. lemming section.
Download
Both light switch models are included within the Matlab Toolbox and can be downloaded there.
References
[1] [http://jb.asm.org/cgi/reprint/183/21/6365?maxtoshow=&hits=10&RESULTFORMAT=1&andorexacttitle=and&andorexacttitleabs=and&fulltext=An+archaeal+photosignal-transducing+module+mediates+phototaxis+in+%27%27Escherichia+&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT: Jung et al: An Archaeal Photosignal-Transducing Module Mediates Phototaxis in Escherichia coli]
 "
"