Team:Korea U Seoul/Notebook
From 2010.igem.org
(→Experiment note) |
(→[ Digestion ] 2010-10-01 ~ 2010-10-03) |
||
| (83 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | {{KUmenu}} | |
| - | {{ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<html> | <html> | ||
<style type="text/css"> | <style type="text/css"> | ||
table.calendar { margin: 0; padding: 2px; } | table.calendar { margin: 0; padding: 2px; } | ||
| - | table.calendar td { margin: 0; padding: 1px; vertical-align: top; } | + | table.calendar td { margin: 0; padding: 1px; vertical-align: top;} |
| - | table.month .heading td { padding:1px; background-color: | + | table.month .heading td { width:300px;padding:1px; background-color:#d0d0d0 ; bordr: 1px ; |
| - | table.month .dow td { color: | + | color: white ; text-align:center; font-size:140%; font-weight:bold; } |
| - | table.month td.today { background-color: | + | table.month .dow td { color:gray; text-align:center; font-size:100%; } |
| + | table.month td.today { background-color:gray;} | ||
table.month td { | table.month td { | ||
border: none; | border: none; | ||
| Line 50: | Line 18: | ||
} | } | ||
#bodyContent table.month a { background:none; padding:0 } | #bodyContent table.month a { background:none; padding:0 } | ||
| - | .day-active { color: | + | .day-active { color:black } |
| - | .day-empty { color:# | + | .day-empty { color:#d0d0d0 } |
</style> | </style> | ||
</html> | </html> | ||
| + | <div id="yong"> | ||
| + | <!--- The Mission, Experiments ---> | ||
| + | ='''Brain storming & Work notes'''= | ||
| + | Click on a date to see notes on the meeting & summary of labwork done on that day. | ||
| - | |||
| - | |||
{| align="center" | {| align="center" | ||
| Line 67: | Line 37: | ||
|align="center" width="150pt"|{{#calendar: title=Korea_U_Seoul |year=2010 | month=09}} | |align="center" width="150pt"|{{#calendar: title=Korea_U_Seoul |year=2010 | month=09}} | ||
|align="center" width="150pt"|{{#calendar: title=Korea_U_Seoul |year=2010 | month=10}} | |align="center" width="150pt"|{{#calendar: title=Korea_U_Seoul |year=2010 | month=10}} | ||
| + | |} | ||
| + | <br> | ||
| + | ='''Experimental notes'''= | ||
| + | |||
| + | == [Discussion] 2010-08-02 ~ 2010-08-29 == | ||
| + | <br/> | ||
| + | 1. Strategy and overview of iGEM 2010 experiment | ||
| + | [[Image:Kuprojectmain.png]] | ||
| + | |||
| + | 2. Design of primers | ||
| + | <br/> | ||
| + | <br/> | ||
| + | {| border="2" | ||
| + | !Primer | ||
| + | !Sequence ( 5’ → 3’ ) | ||
| + | |- | ||
| + | |PyodA(''Eco''RI)_F | ||
| + | |CCGGAATTCCTTCATATTGCCGACAAAGTACG | ||
| + | |- | ||
| + | |mAAA(''Spe''I)_R | ||
| + | |GGACTAGTTTATCACAGGGGCCGTCCG | ||
| + | |- | ||
| + | |PzntA(''Xba''I)_F | ||
| + | |GCTCTAGACGTCCGCTCGCTGTATCTC | ||
| + | |- | ||
| + | |RFP(''Pst''I)_R | ||
| + | |AACTGCAGCGGCCGCTACTAGTTTATTAAGCACCGGTGGAGTGA | ||
| + | |- | ||
| + | |ParsR(''Xba''I)_F | ||
| + | |GCTCTAGACCAACTCAAAATTCACACCTATTAC | ||
| + | |- | ||
| + | |GFP(''Pst''I)_R | ||
| + | |AACTGCAGTTAAGGCCTTTTGTATAGTTCATCC | ||
| + | |} | ||
| + | |||
| + | == [ Preparation of competent cells ] 2010-09-01 ~ 2010-09-03 == | ||
| + | <br/> | ||
| + | 1. Inoculation of ''E. coli'' DH5α and ''E. coli'' BL21(DE3) to 3mL LB broth | ||
| + | |||
| + | 2. Preparation of 200mL 2x LB broth, TSS solution and LB plates with ampicillin(100μg/mL) and chloramphenicol(25μg /mL), respectively | ||
| + | |||
| + | 3. Inoculation of subcultured ''E. coli'' to 200mL 2x LB borth | ||
| + | |||
| + | 4. Preparation of competent cells by CSBL laboratory protocol | ||
| + | |||
| + | 5. Transformation of pUC19 plasmid(10ng/μL) to competent cells for transformation efficiency check | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | == [ Transformation efficiency ] 2010-09-04 == | ||
| + | <br/> | ||
| + | <center> | ||
| + | {| border="2" | ||
| + | ! Strain | ||
| + | ! Number of colonies (colonies/μg DNA) | ||
| + | |- | ||
| + | |''E. coli'' DH5α | ||
| + | |Number of colonies (colonies/μg DNA) | ||
| + | |- | ||
| + | |''E. coli'' BL21(DE3) | ||
| + | |1.5 x 105 | ||
| + | |} | ||
| + | </center> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | == [ Amplification of BioBrick parts : pSB1A2 and pSB1C3 ] 2010-09-05 == | ||
| + | <br/> | ||
| + | 1. Confirmed location : pSB1A2-BBa_E0040 (2010 Kit plate 1/ 14K) and pSB1C3-BBa_J04450 (2010 Kit plate 1/ 3A) | ||
| + | |||
| + | 2. 20uL suspension by autoclaved distilled water | ||
| + | |||
| + | 3. 3uL transformation to ''E. coli'' DH5α | ||
| + | |||
| + | 4. Plating to LB(Amp100), LB(Cm25) | ||
| + | <br/> | ||
| + | <br/> | ||
| + | == [ Genomic DNA extraction ] 2010-09-06 == | ||
| + | <br/> | ||
| + | 1. Inoculation for plasmid DNA purification | ||
| + | |||
| + | 2. ''E. coli'' K12 genomic DNA extraction by AccuPrep® Genomic DNA Extraction Kit | ||
| + | |||
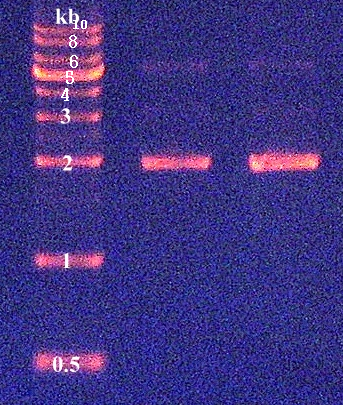
| + | 3. Confirmation of genomic DNA by agarose gel electrophoresis (Figure 1) | ||
| + | |||
| + | 4. Quantification of DNA concentration by NanoDrop : 137.5ng/μL | ||
| + | |||
| + | |||
| + | [[Image:Real_figure_1.jpg|left|140px|frame|figure1. lane1;M-lane2;''E. coli'' K12 genomic DNA extraction]] | ||
| + | |||
| + | |||
| + | <br><br><br><br><br><br><br><br><br>br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | |||
| + | == [ Plasmid DNA extraction : pSB1A3 and pSB1C3 ] 2010-09-07== | ||
| + | <br/> | ||
| + | 1. Plasmid miniprep by LaboPass™ Plasmid Mini (Plasmid DNA purification kit) | ||
| + | |||
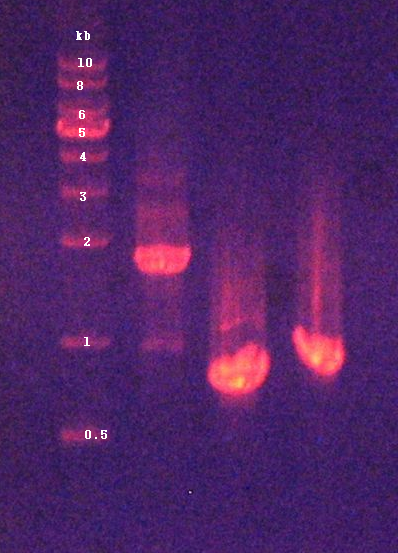
| + | 2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 2) | ||
| + | |||
| + | 3. Quantification of DNA concentration by NanoDrop | ||
| + | [[Image:Figure1_실험.png |250px|left|frame|figure2.'''lane1;M-lane2;pSB1A2-lane3;pSB1C3''']] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <br><br> | ||
| + | <br><br><br> | ||
| + | <br> | ||
| + | <br><br> | ||
| + | <br> | ||
| + | <br><br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | == [ PCR : promoters and reporter genes ] 2010-09-13 ~ 2010-09-16== | ||
| + | <br/> | ||
| + | 1. PCR : PyodA-mAAA, PzntA-RFP(BBa_E1010) and ParsR-GFP(BBa_E0040) | ||
| + | <br/> | ||
| + | {| border="2" | ||
| + | ! Reagent | ||
| + | ! Volume (μL) | ||
| + | |- | ||
| + | |2.5mM dNTP | ||
| + | |3 | ||
| + | |- | ||
| + | |10x buffer | ||
| + | |5 | ||
| + | |- | ||
| + | |Plasmid template (20ng/μL) | ||
| + | |2 | ||
| + | |- | ||
| + | |Primers (10pmole/μL) | ||
| + | |4 | ||
| + | |- | ||
| + | |α-Taq DNA polymerase (5U/μL) | ||
| + | |0.5 | ||
| + | |- | ||
| + | |D.W. | ||
| + | |35.5/total=50 | ||
| + | |- | ||
| + | !95˚C(2’)-[95˚C(20”)-55˚C(20”)-72˚C(2’)]30-72˚C(5’)-4˚C | ||
| + | |} | ||
| + | <br/> | ||
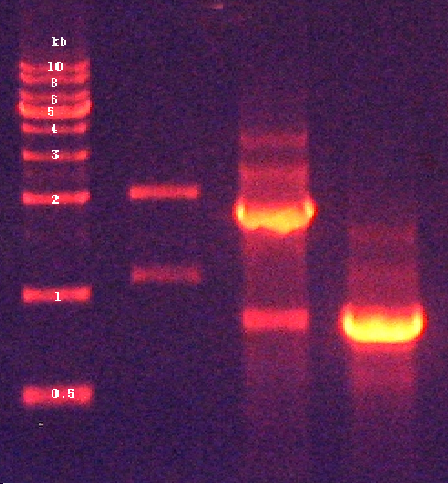
| + | 2. Confirmation of PCR products by agarose gel electrophoresis (Figure 3) | ||
| + | |||
| + | 3. Purified PCR products | ||
| + | |||
| + | 4. Quantification of DNA concentration by NanoDrop | ||
| + | |||
| + | |||
| + | [[Image:Figure2 실험.png|140px|left|frame|figure3.lane1;M-lane2;(PyodA-mAAA)-lane3;(Pznt-RFP)-lane4;(ParsR-GFP)]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/><br/> | ||
| + | |||
| + | == [ Digestion] 2010-09-17 == | ||
| + | 1. Digestion of PCR products and pSB1A2 | ||
| + | |||
| + | :1) PyodA-mAAA : ''Eco''RI and ''Spe''I | ||
| + | |||
| + | :2) PzntA-RFP : ''Xba''I and ''Pst''I | ||
| + | |||
| + | :3) pSB1A3 : ''Eco''RI and ''Pst''I | ||
| + | <br/> | ||
| + | {| border="2" | ||
| + | ! Reagent | ||
| + | ! Volume (μL) | ||
| + | |- | ||
| + | |DNA (about 30ng/μL) | ||
| + | |30 | ||
| + | |- | ||
| + | |10x NEB buffer 2 | ||
| + | |5 | ||
| + | |- | ||
| + | |BSA (10mg/mL) | ||
| + | |0.5 | ||
| + | |- | ||
| + | |Appropriate 1st and 2nd restriction enzymes | ||
| + | |2 (each 1) | ||
| + | |- | ||
| + | |D.W. | ||
| + | |12.5 / total = 50 | ||
| + | |- | ||
| + | !Completely digestion at 37˚C for 2 hours (at least) | ||
| + | and stop at 80˚C for 20min | ||
| + | |} | ||
| + | <br/> | ||
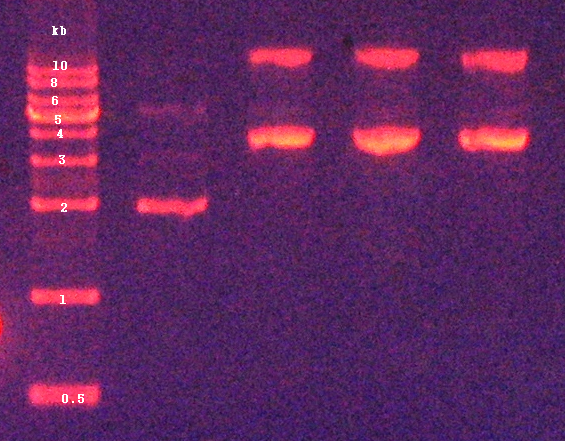
| + | 2. Confirmation of digested products by agarose gel electrophoresis (Figure 4) | ||
| + | |||
| + | 3. Quantification of DNA concentration by NanoDrop | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:4 Figure3 실험.png|left|140px|frame|figure4.M pSB1A2(''Eco''RI/''Pst''I) PyodA-mAAA(''Eco''RI/''Spe''I) PzntA-RFP(''Xba''I/''Pst''I)]] | ||
| + | |||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | == [Chuseok, Korean thanksgiving day] 2010-09-20 ~ 2010-09== | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | == [ Ligation & Transformation ] 2010-09-27== | ||
| + | <br/> | ||
| + | 1. Ligation of each parts : PyodA-mAAA, PzntA-RFP and pSB1A2 | ||
| + | {| border="2" | ||
| + | !Reagent | ||
| + | !Volume (μL) | ||
| + | |- | ||
| + | |10x T4 DNA ligase reaction buffer | ||
| + | |2 | ||
| + | |- | ||
| + | |T4 DNA ligase | ||
| + | |2 | ||
| + | |- | ||
| + | |Each of the digests | ||
| + | |2 + 2 + 2 = 8 | ||
| + | |- | ||
| + | |D.W. | ||
| + | |8 / total = 20 | ||
| + | |- | ||
| + | !Incubation at room temperature for 30min | ||
| + | and stop at 80˚C for 20min | ||
| + | |} | ||
| + | <br/> | ||
| + | 2. Transformation to ''E. coli'' DH5α | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | == [ Confirmation of 1st cloning ] 2010-09-28== | ||
| + | <br/> | ||
| + | 1. Check : the color of colonies '''(pSB1A2 : green, recombinant plasmid : white)''' | ||
| + | |||
| + | 2. Inoculation of white colonies to 3mL LB(Amp100) | ||
| + | <br/> | ||
| + | <br/> | ||
| + | == [ Plasmid DNA extraction : pSB1A2-( PyodA-mAAA-PzntA-RFP) ] 2010-09-29== | ||
| + | <br/> | ||
| + | 1. Plasmid DNA purification by LaboPass™ Plasmid Mini | ||
| + | |||
| + | 2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 5) | ||
| + | |||
| + | 3. Recombinant plasmid sequencing by COSMO GeneTech | ||
| + | |||
| + | [[Image:5_Figure4_실험.png|left|140px|frame|figure5. lane1;M-lane2; (pSB1A2)-[PyodA-mAA-Pznt-RFP] #1,2,3]] | ||
| + | |||
| + | <br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br><br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br><br><br><br><br> | ||
| + | |||
| + | == [ Digestion ] 2010-10-01 ~ 2010-10-03== | ||
| + | <br/> | ||
| + | 1. Check : recombinant plasmid sequence | ||
| + | 2. Selection of correct clones | ||
| + | 3. Digestion of PCR products(ParsR-GFP) and pSB1C3 | ||
| + | :1) PyodA-mAAA-PzntA-RFP : ''Eco''RI and ''Spe''I | ||
| + | :2) ParsR-GFP : ''Eco''RI and ''Spe''I | ||
| + | :3) pSB1C3 : ''Eco''RI and ''Pst''I | ||
| + | <br/> | ||
| + | {| border="2" | ||
| + | !Reagent | ||
| + | !Volume (μL) | ||
| + | |- | ||
| + | |DNA (about 30ng/μL) | ||
| + | |30 | ||
| + | |- | ||
| + | |10x NEB buffer 2 | ||
| + | |5 | ||
| + | |- | ||
| + | |BSA (10mg/mL) | ||
| + | |0.5 | ||
| + | |- | ||
| + | |Appropriate 1st and 2nd restriction enzymes | ||
| + | |2 (each 1) | ||
| + | |- | ||
| + | |D.W. | ||
| + | |12.5 / total = 50 | ||
| + | |- | ||
| + | !Completely digestion at 37˚C for 2 hours (at least) | ||
| + | and stop at 80˚C for 20min | ||
| + | |} | ||
| + | <br/> | ||
| + | 4. Confirmation of digested products by agarose gel electrophoresis (Figure 6) | ||
| + | [[Image:Figure6 aaa.png|left|140px|frame|figure6. lane1; M-lane2; (PyodA-mAAA-PzntA-RFP(EcoRI/SpeI))-lane3; (ParsR-GFP(XbaI/SpeI))-lane4; (pSB1C3(EcoRI/PstI))]] | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | |||
| + | |||
| + | 5. Quantification of DNA concentration by NanoDrop | ||
| + | |||
| + | 6. Ligation of each parts : PyodA-mAAA-PzntA-RFP, ParsR-GFP and pSB1C3 | ||
| + | |||
| + | <br/> | ||
| + | {| border="2" | ||
| + | !Reagent | ||
| + | !Volume (μL) | ||
| + | |- | ||
| + | |10x T4 DNA ligase reaction buffer | ||
| + | |2 | ||
| + | |- | ||
| + | |T4 DNA ligase | ||
| + | |2 | ||
| + | |- | ||
| + | |Each of the digests | ||
| + | |2 + 2 + 2 = 8 | ||
| + | |- | ||
| + | |D.W. | ||
| + | |8 / total = 20 | ||
| + | |- | ||
| + | !Incubation at room temperature for 30min | ||
| + | and stop at 80˚C for 20min | ||
| + | |} | ||
| + | <br/> | ||
| + | 7. Transformation to ''E. coli'' DH5α | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | == [ Confirmation of 2nd cloning ] 2010-10-06== | ||
| + | <br/> | ||
| + | 1. Check : the color of colonies (pSB1C3 : red, recombinant plasmid : white) | ||
| + | |||
| + | 2. Inoculation of white colonies to 3mL LB(Amp100) | ||
| + | <br/> | ||
| + | <br/> | ||
| + | == [ Plasmid DNA extraction : pSB1C3-( PyodA-mAAA-PzntA-RFP-ParsR-GFP) ] 2010-10-07== | ||
| + | <br/> | ||
| + | 1. Plasmid DNA purification by LaboPass™ Plasmid Mini | ||
| + | |||
| + | 2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 7) | ||
| + | |||
| + | 3. Recombinant plasmid full-sequencing by COSMO GeneTech | ||
| + | |||
| + | [[Image:Figure7_실험.png|left|100px|frame|figure7. lane1;M-lane2; (psB1A2) Heavy-metal detector #1~3]] | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | == [ Completion : Heavy-metal detector ] 2010-10-18== | ||
| + | <br/> | ||
| + | 1. Check : recombinant plasmid sequence | ||
| + | |||
| + | 2. Selection of correct clones | ||
| + | |||
| + | 3. Transformation to ''E. coli'' BL21(DE3) for expression test | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | </div> | ||
Latest revision as of 03:58, 28 October 2010

Brain storming & Work notes
Click on a date to see notes on the meeting & summary of labwork done on that day.
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental notes
[Discussion] 2010-08-02 ~ 2010-08-29
1. Strategy and overview of iGEM 2010 experiment
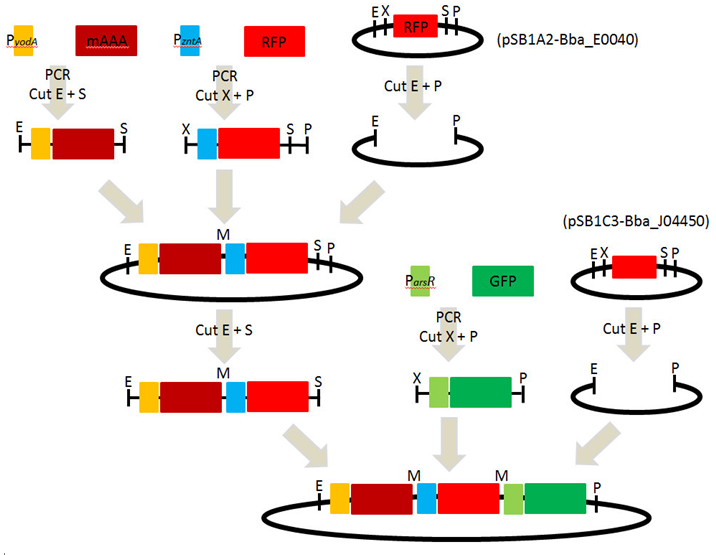
2. Design of primers
| Primer | Sequence ( 5’ → 3’ ) |
|---|---|
| PyodA(EcoRI)_F | CCGGAATTCCTTCATATTGCCGACAAAGTACG |
| mAAA(SpeI)_R | GGACTAGTTTATCACAGGGGCCGTCCG |
| PzntA(XbaI)_F | GCTCTAGACGTCCGCTCGCTGTATCTC |
| RFP(PstI)_R | AACTGCAGCGGCCGCTACTAGTTTATTAAGCACCGGTGGAGTGA |
| ParsR(XbaI)_F | GCTCTAGACCAACTCAAAATTCACACCTATTAC |
| GFP(PstI)_R | AACTGCAGTTAAGGCCTTTTGTATAGTTCATCC |
[ Preparation of competent cells ] 2010-09-01 ~ 2010-09-03
1. Inoculation of E. coli DH5α and E. coli BL21(DE3) to 3mL LB broth
2. Preparation of 200mL 2x LB broth, TSS solution and LB plates with ampicillin(100μg/mL) and chloramphenicol(25μg /mL), respectively
3. Inoculation of subcultured E. coli to 200mL 2x LB borth
4. Preparation of competent cells by CSBL laboratory protocol
5. Transformation of pUC19 plasmid(10ng/μL) to competent cells for transformation efficiency check
[ Transformation efficiency ] 2010-09-04
| Strain | Number of colonies (colonies/μg DNA) |
|---|---|
| E. coli DH5α | Number of colonies (colonies/μg DNA) |
| E. coli BL21(DE3) | 1.5 x 105 |
[ Amplification of BioBrick parts : pSB1A2 and pSB1C3 ] 2010-09-05
1. Confirmed location : pSB1A2-BBa_E0040 (2010 Kit plate 1/ 14K) and pSB1C3-BBa_J04450 (2010 Kit plate 1/ 3A)
2. 20uL suspension by autoclaved distilled water
3. 3uL transformation to E. coli DH5α
4. Plating to LB(Amp100), LB(Cm25)
[ Genomic DNA extraction ] 2010-09-06
1. Inoculation for plasmid DNA purification
2. E. coli K12 genomic DNA extraction by AccuPrep® Genomic DNA Extraction Kit
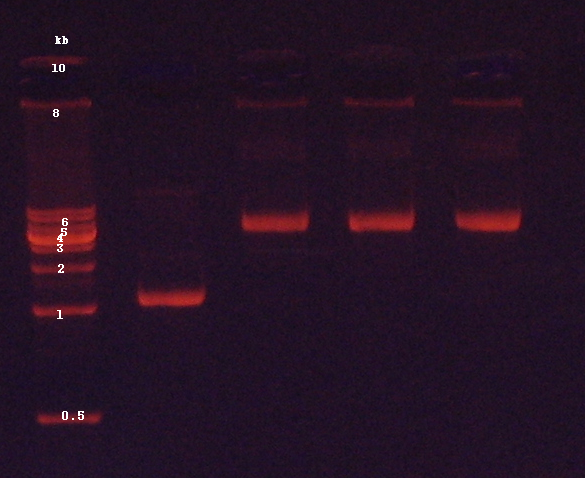
3. Confirmation of genomic DNA by agarose gel electrophoresis (Figure 1)
4. Quantification of DNA concentration by NanoDrop : 137.5ng/μL
br>
[ Plasmid DNA extraction : pSB1A3 and pSB1C3 ] 2010-09-07
1. Plasmid miniprep by LaboPass™ Plasmid Mini (Plasmid DNA purification kit)
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 2)
3. Quantification of DNA concentration by NanoDrop
[ PCR : promoters and reporter genes ] 2010-09-13 ~ 2010-09-16
1. PCR : PyodA-mAAA, PzntA-RFP(BBa_E1010) and ParsR-GFP(BBa_E0040)
| Reagent | Volume (μL) |
|---|---|
| 2.5mM dNTP | 3 |
| 10x buffer | 5 |
| Plasmid template (20ng/μL) | 2 |
| Primers (10pmole/μL) | 4 |
| α-Taq DNA polymerase (5U/μL) | 0.5 |
| D.W. | 35.5/total=50 |
| 95˚C(2’)-[95˚C(20”)-55˚C(20”)-72˚C(2’)]30-72˚C(5’)-4˚C |
2. Confirmation of PCR products by agarose gel electrophoresis (Figure 3)
3. Purified PCR products
4. Quantification of DNA concentration by NanoDrop
[ Digestion] 2010-09-17
1. Digestion of PCR products and pSB1A2
- 1) PyodA-mAAA : EcoRI and SpeI
- 2) PzntA-RFP : XbaI and PstI
- 3) pSB1A3 : EcoRI and PstI
| Reagent | Volume (μL) |
|---|---|
| DNA (about 30ng/μL) | 30 |
| 10x NEB buffer 2 | 5 |
| BSA (10mg/mL) | 0.5 |
| Appropriate 1st and 2nd restriction enzymes | 2 (each 1) |
| D.W. | 12.5 / total = 50 |
| Completely digestion at 37˚C for 2 hours (at least)
and stop at 80˚C for 20min |
2. Confirmation of digested products by agarose gel electrophoresis (Figure 4)
3. Quantification of DNA concentration by NanoDrop
[Chuseok, Korean thanksgiving day] 2010-09-20 ~ 2010-09
[ Ligation & Transformation ] 2010-09-27
1. Ligation of each parts : PyodA-mAAA, PzntA-RFP and pSB1A2
| Reagent | Volume (μL) |
|---|---|
| 10x T4 DNA ligase reaction buffer | 2 |
| T4 DNA ligase | 2 |
| Each of the digests | 2 + 2 + 2 = 8 |
| D.W. | 8 / total = 20 |
| Incubation at room temperature for 30min
and stop at 80˚C for 20min |
2. Transformation to E. coli DH5α
[ Confirmation of 1st cloning ] 2010-09-28
1. Check : the color of colonies (pSB1A2 : green, recombinant plasmid : white)
2. Inoculation of white colonies to 3mL LB(Amp100)
[ Plasmid DNA extraction : pSB1A2-( PyodA-mAAA-PzntA-RFP) ] 2010-09-29
1. Plasmid DNA purification by LaboPass™ Plasmid Mini
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 5)
3. Recombinant plasmid sequencing by COSMO GeneTech
[ Digestion ] 2010-10-01 ~ 2010-10-03
1. Check : recombinant plasmid sequence
2. Selection of correct clones
3. Digestion of PCR products(ParsR-GFP) and pSB1C3
- 1) PyodA-mAAA-PzntA-RFP : EcoRI and SpeI
- 2) ParsR-GFP : EcoRI and SpeI
- 3) pSB1C3 : EcoRI and PstI
| Reagent | Volume (μL) |
|---|---|
| DNA (about 30ng/μL) | 30 |
| 10x NEB buffer 2 | 5 |
| BSA (10mg/mL) | 0.5 |
| Appropriate 1st and 2nd restriction enzymes | 2 (each 1) |
| D.W. | 12.5 / total = 50 |
| Completely digestion at 37˚C for 2 hours (at least)
and stop at 80˚C for 20min |
4. Confirmation of digested products by agarose gel electrophoresis (Figure 6)
5. Quantification of DNA concentration by NanoDrop
6. Ligation of each parts : PyodA-mAAA-PzntA-RFP, ParsR-GFP and pSB1C3
| Reagent | Volume (μL) |
|---|---|
| 10x T4 DNA ligase reaction buffer | 2 |
| T4 DNA ligase | 2 |
| Each of the digests | 2 + 2 + 2 = 8 |
| D.W. | 8 / total = 20 |
| Incubation at room temperature for 30min
and stop at 80˚C for 20min |
7. Transformation to E. coli DH5α
[ Confirmation of 2nd cloning ] 2010-10-06
1. Check : the color of colonies (pSB1C3 : red, recombinant plasmid : white)
2. Inoculation of white colonies to 3mL LB(Amp100)
[ Plasmid DNA extraction : pSB1C3-( PyodA-mAAA-PzntA-RFP-ParsR-GFP) ] 2010-10-07
1. Plasmid DNA purification by LaboPass™ Plasmid Mini
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 7)
3. Recombinant plasmid full-sequencing by COSMO GeneTech
[ Completion : Heavy-metal detector ] 2010-10-18
1. Check : recombinant plasmid sequence
2. Selection of correct clones
3. Transformation to E. coli BL21(DE3) for expression test
 "
"