Team:Kyoto/Notebook1
From 2010.igem.org
(→Electrophoresis) |
(→Ligation and Transformation of LTCS) |
||
| (93 intermediate revisions not shown) | |||
| Line 73: | Line 73: | ||
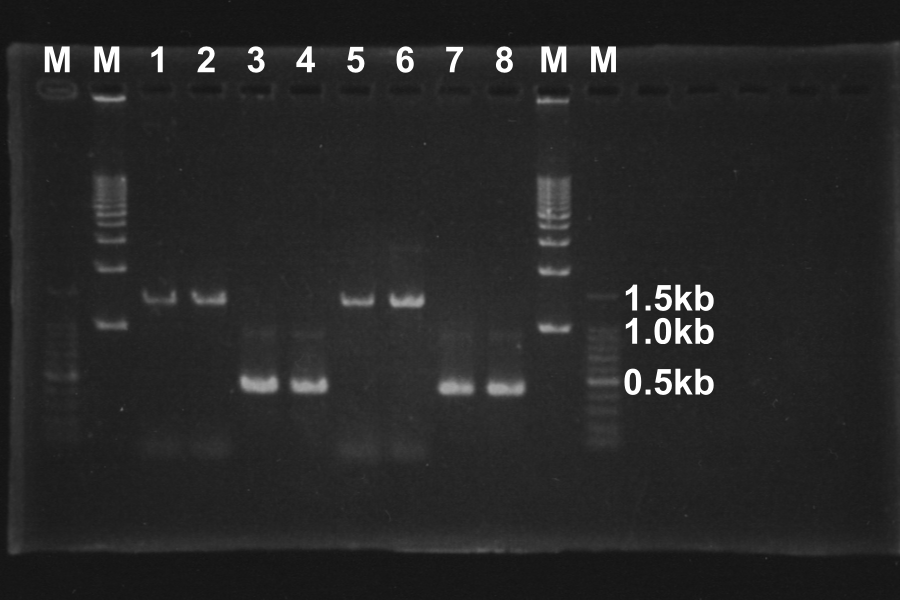
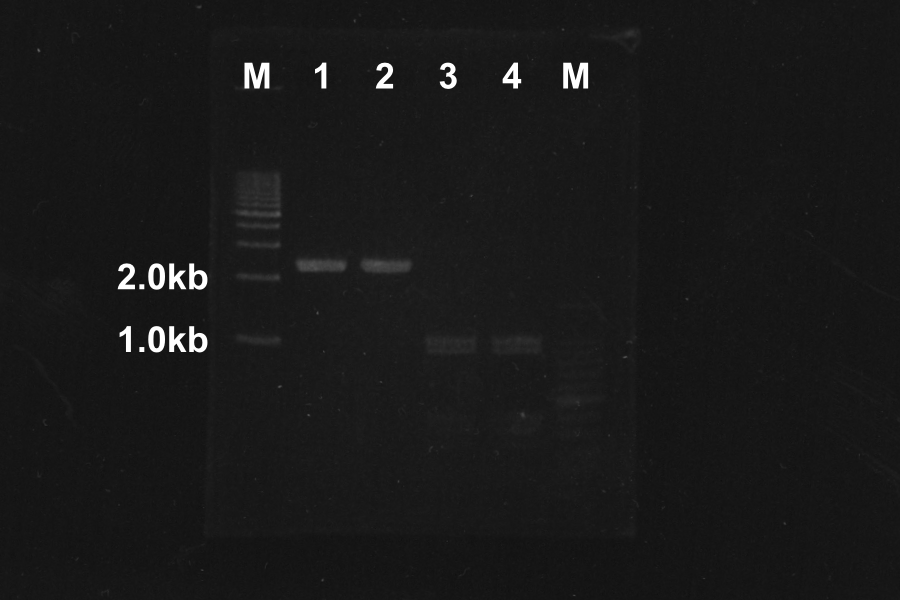
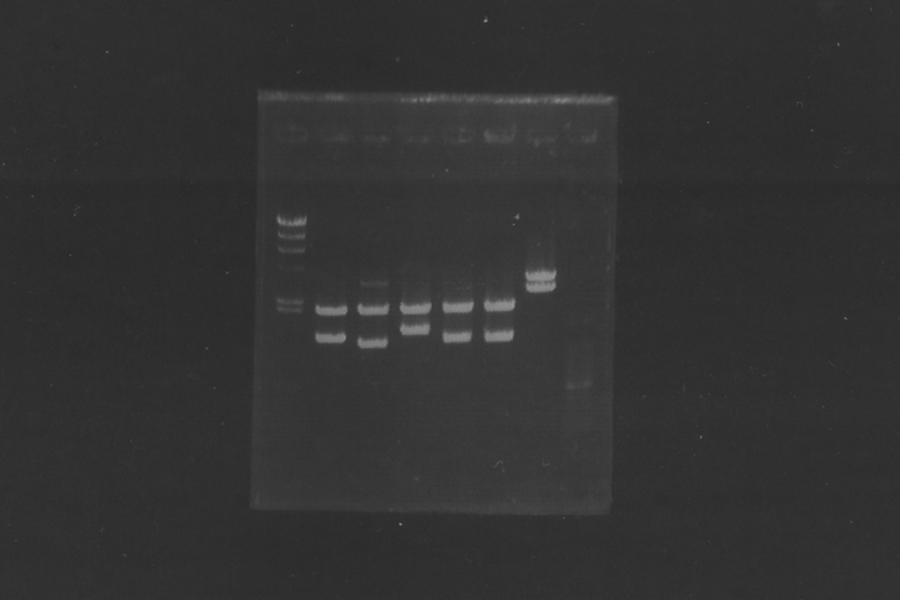
=====Electrophoresis (40min) of the PCR Products===== | =====Electrophoresis (40min) of the PCR Products===== | ||
[[Image:KyotoExp100722-1.png|300px|right]] | [[Image:KyotoExp100722-1.png|300px|right]] | ||
| - | + | * Sample: 1, 2, 5, 6 = SRRz, 3, 4, 7, 8 = S | |
| - | + | * Marker: 100bp, 1kb, 1kb, 100bp. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | Marker: 100bp, 1kb, 1kb, 100bp. | + | |
=====Miniprep===== | =====Miniprep===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 244: | Line 227: | ||
| - | ====Tuesday, July 27 <span class="by">By: Wataru, Tomo, Kazuya, Ken, Naoi==== | + | ====Tuesday, July 27 <span class="by">By: Wataru, Tomo, Kazuya, Ken, Naoi</span>==== |
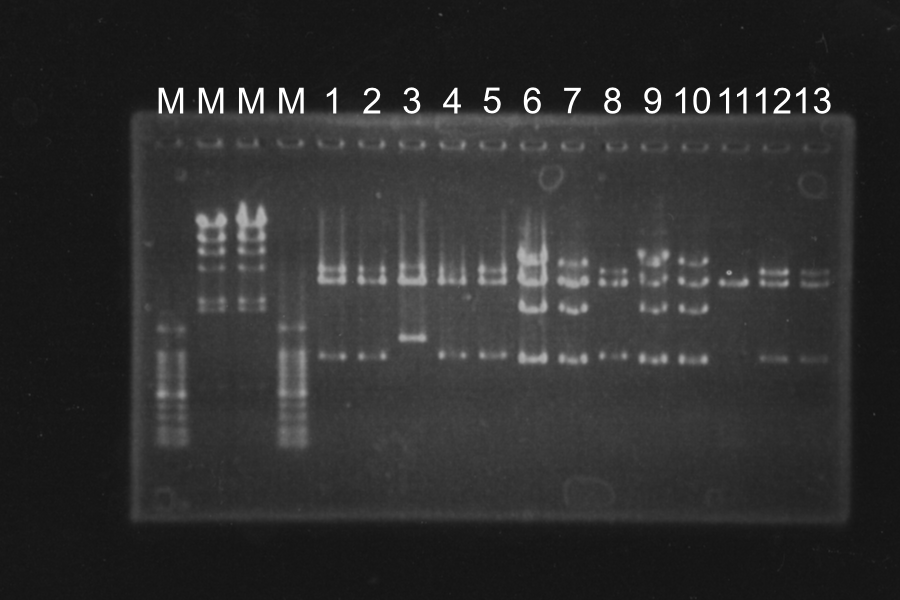
=====Colony PCR of S<sub>Sam7</sub>-E0840 (Electrophoresis for 35min)===== | =====Colony PCR of S<sub>Sam7</sub>-E0840 (Electrophoresis for 35min)===== | ||
[[Image:KyotoExp100727-1.png|300px|right]] | [[Image:KyotoExp100727-1.png|300px|right]] | ||
| Line 530: | Line 513: | ||
!Name||Vector||colspan="2"|Insert 1||colspan="2"|Insert 2||Ligation High||T4 Kinase||Total||Incubation | !Name||Vector||colspan="2"|Insert 1||colspan="2"|Insert 2||Ligation High||T4 Kinase||Total||Incubation | ||
|- | |- | ||
| - | | | + | |ML (1)||pSB4K5 [EP]||1||R0011 [ES]||1||E0240(1) [XP]||1||3||15||rowspan="2"|17:30 - 20:20 |
|- | |- | ||
| - | | | + | |ML (2)||pSB4K5 [EP]||1||R0011 [ES]||1||E0240(2) [XP]||1||3||15 |
|} | |} | ||
| Line 577: | Line 560: | ||
!||Vector||colspan="2"|Insert||colspan="2"|Ligation High||Total||Incubation | !||Vector||colspan="2"|Insert||colspan="2"|Ligation High||Total||Incubation | ||
|- | |- | ||
| - | | | + | |MS||pSB4K5 [EP]||1||I20260 [EP]||1||2||4||20:00-20:30 |
|} | |} | ||
<div class="measure-construction"> | <div class="measure-construction"> | ||
| Line 584: | Line 567: | ||
!Name||Sample||Competent Cell||Total||Plate||Incubation||Colony | !Name||Sample||Competent Cell||Total||Plate||Incubation||Colony | ||
|- | |- | ||
| - | | | + | |ML (1)||1 µL||20||21||rowspan="3"|LB (Kan+)||rowspan="3"|08/03-08/04||○ |
|- | |- | ||
| - | | | + | |ML (2)||1||20||21||○ |
|- | |- | ||
| - | | | + | |MS||1||20||21||○ |
|} | |} | ||
| Line 596: | Line 579: | ||
pSB4K5 is inserted RFP generator. We didn't distinguish this inserted parts from low copy plasmid backbone, so self-ligated colony is red. So, white colony is correctly inserted parts. | pSB4K5 is inserted RFP generator. We didn't distinguish this inserted parts from low copy plasmid backbone, so self-ligated colony is red. So, white colony is correctly inserted parts. | ||
| - | However, white colonies and green colonies are observed in | + | However, white colonies and green colonies are observed in ML (1) and ML (2) plate. We cultured both white and green colonies. |
| - | In | + | In MS, Many of colonies are red, but green colonies are observed. We cultured green colonies. |
{| class="experiments" | {| class="experiments" | ||
!Name||Color||Incubation | !Name||Color||Incubation | ||
|- | |- | ||
| - | | | + | |ML (1-1)||Green Colony||rowspan="11"|8/5-8/6 |
|- | |- | ||
| - | | | + | |ML (1-2)||Green Colony |
|- | |- | ||
| - | | | + | |ML (1-3)||White Colony |
|- | |- | ||
| - | | | + | |ML (1-4)||White Colony |
|- | |- | ||
| - | | | + | |ML (2-1)||Green Colony |
|- | |- | ||
| - | | | + | |ML (2-2)||White Colony |
|- | |- | ||
| - | | | + | |ML (2-3)||White Colony |
|- | |- | ||
| - | | | + | |ML (2-4)||White Colony |
|- | |- | ||
| - | | | + | |MS (1)||Green Colony |
|- | |- | ||
| - | | | + | |MS (2)||Green Colony |
|- | |- | ||
| - | | | + | |MS (3)||Green Colony |
|} | |} | ||
=====Sequence===== | =====Sequence===== | ||
| Line 644: | Line 627: | ||
!Name | !Name | ||
|- | |- | ||
| - | | | + | |ML (1-1) |
|- | |- | ||
| - | | | + | |ML (1-2) |
|- | |- | ||
| - | | | + | |ML (1-3) |
|- | |- | ||
| - | | | + | |ML (1-4) |
|- | |- | ||
| - | | | + | |ML (2-1) |
|- | |- | ||
| - | | | + | |ML (2-2) |
|- | |- | ||
| - | | | + | |ML (2-3) |
|- | |- | ||
| - | | | + | |ML (2-4) |
|- | |- | ||
| - | | | + | |MS (1) |
|- | |- | ||
| - | | | + | |MS (2) |
|- | |- | ||
| - | | | + | |MS (3) |
|} | |} | ||
=====Restriction Digestion===== | =====Restriction Digestion===== | ||
| Line 670: | Line 653: | ||
!Name||Sample||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | !Name||Sample||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | ||
|- | |- | ||
| - | | | + | |ML (1-1) [EP]||50 µL||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (1-2) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (1-3) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (1-4) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (2-1) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (2-2) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (2-3) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |ML (2-4) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |MS (1) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |MS (2) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|- | |- | ||
| - | | | + | |MS (3) [EP]||50||6||0.6||EcoRI||0.3||PstI||0.3||2.8||60 |
|} | |} | ||
=====Electrophoresis===== | =====Electrophoresis===== | ||
| Line 697: | Line 680: | ||
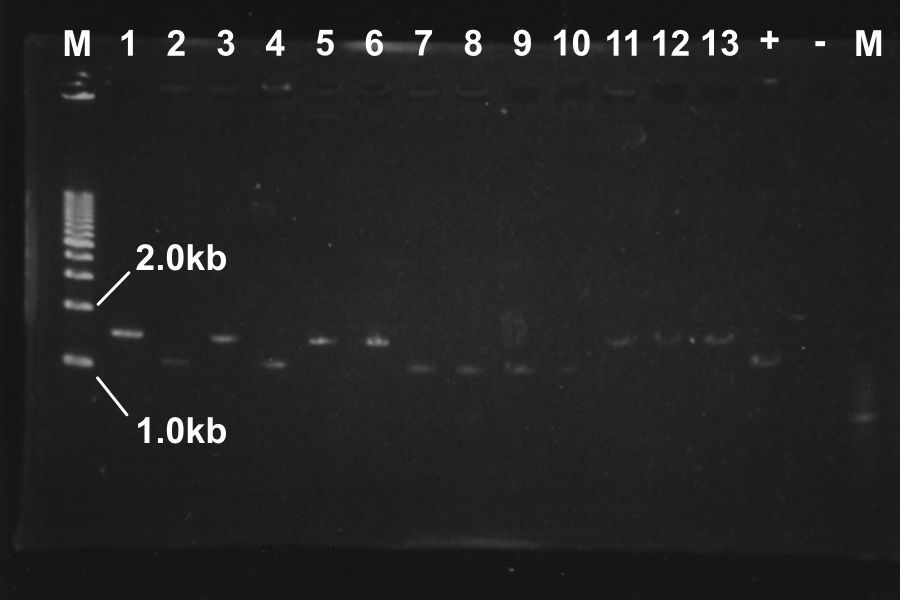
!No.||Name||Length||Results | !No.||Name||Length||Results | ||
|- | |- | ||
| - | |1|| | + | |1||MS (1) [EP]||960, 4339|| |
|- | |- | ||
| - | |2|| | + | |2||MS (2) [EP]||960, 4339|| |
|- | |- | ||
| - | |3|| | + | |3||MS (3) [EP]||960, 4339|| |
|- | |- | ||
| - | |4|| | + | |4||ML (1-1) [EP]||980 3378||○ |
|- | |- | ||
| - | |5|| | + | |5||ML (1-2) [EP]||980 3378||○ |
|- | |- | ||
| - | |6|| | + | |6||ML (1-3) [EP]||980 3378||× |
|- | |- | ||
| - | |7|| | + | |7||ML (1-4) [EP]||980 3378||× |
|- | |- | ||
| - | |8|| | + | |8||ML (2-1) [EP]||980 3378||○ |
|- | |- | ||
| - | |9|| | + | |9||ML (2-2) [EP]||980 3378||× |
|- | |- | ||
| - | |10|| | + | |10||ML (2-3) [EP]||980 3378||× |
|- | |- | ||
| - | |11|| | + | |11||ML (2-4) [EP]||980 3378||× |
|- | |- | ||
| - | |12|| | + | |12||MS (1) [EP]||960, 4339||○ |
|- | |- | ||
| - | |13|| | + | |13||MS (2) [EP]||960, 4339||○ |
|} | |} | ||
White colonies are not inserted R0011 but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of ''lac''I gene. | White colonies are not inserted R0011 but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of ''lac''I gene. | ||
| Line 766: | Line 749: | ||
!Name||concentration | !Name||concentration | ||
|- | |- | ||
| - | | | + | |MS||116.2 ng/µL |
|- | |- | ||
| - | | | + | |ML||146.6 |
|} | |} | ||
=====Transfotrmation===== | =====Transfotrmation===== | ||
| Line 774: | Line 757: | ||
!Sample||Sample||colspan="2"|Competent Cell||Total||Plate||Incuvation||Results | !Sample||Sample||colspan="2"|Competent Cell||Total||Plate||Incuvation||Results | ||
|- | |- | ||
| - | | | + | |MS||2 µL||KRX||50||52||rowspan="2"|LB (Kan+)||rowspan="2"|08/09 18:00-08/10 12:00||○ |
|- | |- | ||
| - | | | + | |ML||2||KRX||50||52||○ |
|} | |} | ||
=====Restriction Eigestion and Ethanol Precipitation===== | =====Restriction Eigestion and Ethanol Precipitation===== | ||
To use R0011 for next ligation, we digested it by EcoRI and PstI | To use R0011 for next ligation, we digested it by EcoRI and PstI | ||
{| class="experiments" | {| class="experiments" | ||
| - | !Name||Sample||10x Buffer||BSA|| | + | !Name||Sample||10x Buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total||Incubation |
|- | |- | ||
|R0011 [EP]||50||6||0.6||EcoRI||0.5||PstI||0.5||2.4||60||At 37℃ 08/09 16:20-18:20 | |R0011 [EP]||50||6||0.6||EcoRI||0.5||PstI||0.5||2.4||60||At 37℃ 08/09 16:20-18:20 | ||
|} | |} | ||
After restriction enzyme digestion, we did ethanol precipitation. | After restriction enzyme digestion, we did ethanol precipitation. | ||
| + | |||
=====Ligation and Transformation===== | =====Ligation and Transformation===== | ||
{| class="experiments" | {| class="experiments" | ||
| - | !Name|Sample||colspan="2"|Competent cell||Total||Plate||Incuvation||Colony | + | !Name||Sample||colspan="2"|Competent cell||Total||Plate||Incuvation||Colony |
|- | |- | ||
|R0011 [pSB4K5, KRX]||2 µL||KRX||50||52||rowspan="2"|LB (Kan+)||rowspan="2"|08/09 20:00-08/10 09:00||○ | |R0011 [pSB4K5, KRX]||2 µL||KRX||50||52||rowspan="2"|LB (Kan+)||rowspan="2"|08/09 20:00-08/10 09:00||○ | ||
| Line 796: | Line 780: | ||
| - | ====Tuesday, August 10 <span class="by">By: Wataru, Tomonori, Ken, Fumitaka</span> | + | ====Tuesday, August 10 <span class="by">By: Wataru, Tomonori, Ken, Fumitaka</span>==== |
| + | |||
=====Culture===== | =====Culture===== | ||
| - | Cultured I20260 [pSB4K5, | + | Cultured I20260 [pSB4K5, ML, R0011 [pSB4K5, KRX], and R0011 [pSB4K5</partrinfo>, C2]. |
=====Minprep===== | =====Minprep===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 868: | Line 853: | ||
|N||-||- | |N||-||- | ||
|} | |} | ||
| - | + | [[image:KyotoExp100811-1.png|300px|right]] | |
{| class="electrophoresis" | {| class="electrophoresis" | ||
!No.||Name||Length||Results | !No.||Name||Length||Results | ||
| Line 888: | Line 873: | ||
|8||R0011 [pSB4K5, C2] (2-N)|||| | |8||R0011 [pSB4K5, C2] (2-N)|||| | ||
|} | |} | ||
| - | |||
Each enzyme correctly cut samples. | Each enzyme correctly cut samples. | ||
| + | |||
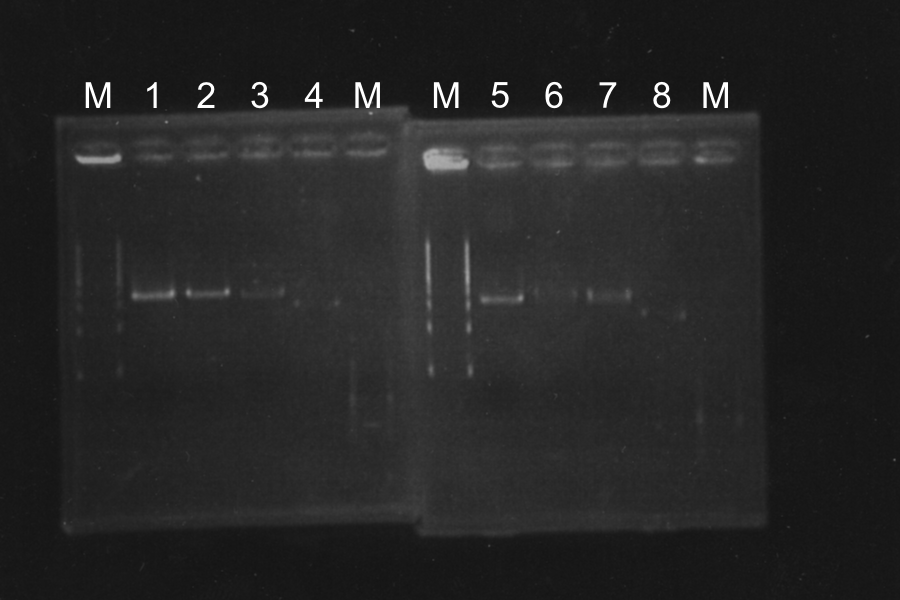
=====Screening PCR of SRRz===== | =====Screening PCR of SRRz===== | ||
| + | [[image:KyotoExp100811-2.png|300px|right]] | ||
| + | [[image:KyotoExp100811-3.png|300px|right]] | ||
{| class="electrophoresis" | {| class="electrophoresis" | ||
!No.||Name||Results | !No.||Name||Results | ||
| Line 905: | Line 892: | ||
|} | |} | ||
Marker: Lambda Marker | Marker: Lambda Marker | ||
| - | + | ||
| - | + | ||
Discussion: All of the sample were self-ligation of DT. SRRz weren't inserted. | Discussion: All of the sample were self-ligation of DT. SRRz weren't inserted. | ||
| Line 933: | Line 919: | ||
| - | ====Thursday, August 19 <span class="by">By: Wataru, Tomo, Ken</span> | + | ====Thursday, August 19 <span class="by">By: Wataru, Tomo, Ken</span>==== |
| + | |||
=====Miniprep of S<sub>Sam7,ΔTMD1</sub>-E0840===== | =====Miniprep of S<sub>Sam7,ΔTMD1</sub>-E0840===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 963: | Line 950: | ||
=====Restriction Digestion by DpnI from 17:50 to 18:50===== | =====Restriction Digestion by DpnI from 17:50 to 18:50===== | ||
=====Electrophoresis===== | =====Electrophoresis===== | ||
| - | {| class="electrophoresis | + | [[Image:KyotoExp100819-1.png|300px|right]] |
| + | {| class="electrophoresis" | ||
!Name | !Name | ||
|- | |- | ||
| Line 973: | Line 961: | ||
|} | |} | ||
Marker: Lambda, 100bp | Marker: Lambda, 100bp | ||
| - | + | ||
=====Ligation and Transformation===== | =====Ligation and Transformation===== | ||
{| class="experiments | {| class="experiments | ||
| Line 1,022: | Line 1,010: | ||
|} | |} | ||
=====Electrophoresis===== | =====Electrophoresis===== | ||
| + | [[Image:KyotoExp100820-1.png|300px|right]] | ||
{| class="electrophoresis" | {| class="electrophoresis" | ||
!Name | !Name | ||
| Line 1,037: | Line 1,026: | ||
|SRRz<sub>Sam7</sub> (6) | |SRRz<sub>Sam7</sub> (6) | ||
|} | |} | ||
| - | |||
Discussion: Primer F1 might be better than F2, because the bands of 1, 2 and 3 were clearer. We decided to use sample 1 and 3. Their bands were clearer in the three. | Discussion: Primer F1 might be better than F2, because the bands of 1, 2 and 3 were clearer. We decided to use sample 1 and 3. Their bands were clearer in the three. | ||
| + | |||
=====PCR Purification===== | =====PCR Purification===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,080: | Line 1,069: | ||
|} | |} | ||
=====Sequence===== | =====Sequence===== | ||
| - | Sample: S<sub>ΔTMD1</sub>-E0840 (1-1). S<sub>ΔTMD1</sub>-E0840 (2-2), | + | Sample: S<sub>ΔTMD1</sub>-E0840 (1-1). S<sub>ΔTMD1</sub>-E0840 (2-2), MS |
| - | Discussion: The sequencing was in success and the results were desirable. It meant the point mutation was succeeded and sequence of | + | Discussion: The sequencing was in success and the results were desirable. It meant the point mutation was succeeded and sequence of MS was confirmed. We decided to use S<sub>ΔTMD1</sub>-E0840. |
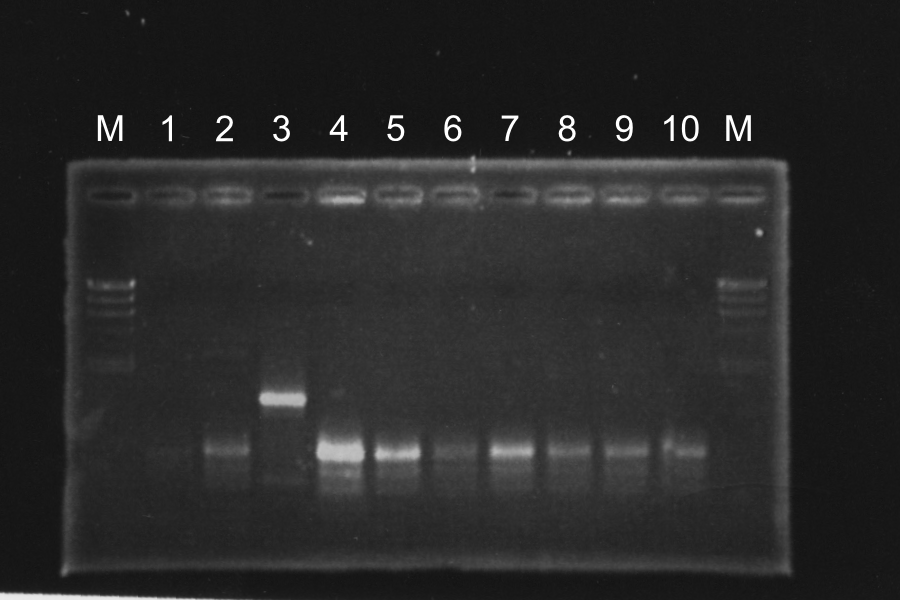
=====Screening PCR of SRRz<sub>Sam7</sub>-B0015===== | =====Screening PCR of SRRz<sub>Sam7</sub>-B0015===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,096: | Line 1,085: | ||
|4℃||hold|| | |4℃||hold|| | ||
|} | |} | ||
| - | + | [[Image:KyotoExp100823-1.png|300px|right]] | |
{| class="electrophoresis" | {| class="electrophoresis" | ||
!No.||Name | !No.||Name | ||
| Line 1,107: | Line 1,096: | ||
|} | |} | ||
Marker: Lambda, 100bp | Marker: Lambda, 100bp | ||
| - | + | ||
Discussion: We found the band; about 200bp, and it meant the lligation was completed successfully. | Discussion: We found the band; about 200bp, and it meant the lligation was completed successfully. | ||
| + | |||
=====Deletion PCR of S<sub>ΔTMD1</sub>-E0840 (2-2)===== | =====Deletion PCR of S<sub>ΔTMD1</sub>-E0840 (2-2)===== | ||
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||10xBuffer||dNTPs||Primer Fwd.||Primer Rev.||Template||Water||KOD-plus-||Total | !Name||Sample||10xBuffer||dNTPs||Primer Fwd.||Primer Rev.||Template||Water||KOD-plus-||Total | ||
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (1)|| | + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||2 µL||5||5||1.5||1.5||1||35||1||50 |
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (2)||5||5||1.5||1.5||1||35||1||50 | + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||2||5||5||1.5||1.5||1||35||1||50 |
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (Control)||5||5||1.5||1.5||1||35||-||50 | + | |rrS<sub>ΔTMD1</sub>-E0840 (Control)||2||5||5||1.5||1.5||1||35||-||50 |
|} | |} | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,140: | Line 1,130: | ||
!Name||Sample||Water||Ligation high||T4 Kinase||Total||Incubation | !Name||Sample||Water||Ligation high||T4 Kinase||Total||Incubation | ||
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (1)||3 µL||6||5||1||15||20:15-21:15 | + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||3 µL||6||5||1||15||rowspan="3"|20:15-21:15 |
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (2)||3||6||5||1|| | + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||3||6||5||1||15 |
|- | |- | ||
|rrS<sub>ΔTMD1</sub>-E0840 (Control)||3||6||5||1||15 | |rrS<sub>ΔTMD1</sub>-E0840 (Control)||3||6||5||1||15 | ||
|} | |} | ||
=====Transformation===== | =====Transformation===== | ||
| - | |||
====Tuesday, August 24 <span class="by">By: Ken, Tomo, Tasuku, Takuya</span>==== | ====Tuesday, August 24 <span class="by">By: Ken, Tomo, Tasuku, Takuya</span>==== | ||
| Line 1,154: | Line 1,143: | ||
!Name||Sample||10xBuffer||dNTPs||MgSO4||Primer1||Primer2||Template||Water||KOD-plus-||Total | !Name||Sample||10xBuffer||dNTPs||MgSO4||Primer1||Primer2||Template||Water||KOD-plus-||Total | ||
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (1)|| | + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||2 µL||5||5||3||1.5||1.5||1||32||1||50 |
|- | |- | ||
| - | |rrS<sub>ΔTMD1</sub>-E0840 (2)||5||5||3||1.5||1.5||1||32||1||50 | + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||2||5||5||3||1.5||1.5||1||32||1||50 |
|- | |- | ||
| - | |Control||5||5||3||1.5||1.5||1||32||1||50 | + | |Control||2||5||5||3||1.5||1.5||1||32||1||50 |
|} | |} | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,174: | Line 1,163: | ||
14:15-15:15 | 14:15-15:15 | ||
=====Electrophoreis===== | =====Electrophoreis===== | ||
| + | [[Image:KyotoExp100824-1.png|300px|right]] | ||
{| class="electrophoresis" | {| class="electrophoresis" | ||
!Lane||Name | !Lane||Name | ||
| Line 1,184: | Line 1,174: | ||
|} | |} | ||
Marker: 100bp, Lambda. | Marker: 100bp, Lambda. | ||
| - | + | ||
We found the band of sample 1 and 2 about 3000bp and there wasn't the band of sample control. So, we confirmed the PCR and Restriction Digestion were completed successfully. | We found the band of sample 1 and 2 about 3000bp and there wasn't the band of sample control. So, we confirmed the PCR and Restriction Digestion were completed successfully. | ||
=====Ligation===== | =====Ligation===== | ||
| Line 1,211: | Line 1,201: | ||
[[Image:KyotoExp100824-2.png|300px|right]] | [[Image:KyotoExp100824-2.png|300px|right]] | ||
We could find point mutation PCR and restriction enzyme of DpnI was done. | We could find point mutation PCR and restriction enzyme of DpnI was done. | ||
| - | ====PCR of E0240==== | + | |
| + | =====PCR of E0240===== | ||
{| class="experiments" | {| class="experiments" | ||
!Sample||10xBuffer||dNTPs||MgSO4||VF2||VR||Template||Water||KOD-plus-||Total | !Sample||10xBuffer||dNTPs||MgSO4||VF2||VR||Template||Water||KOD-plus-||Total | ||
| Line 1,238: | Line 1,229: | ||
=====Transformation===== | =====Transformation===== | ||
| - | + | ====Wednesday, August 25 <span class="by">By:Ken, Tomo, Kazuya, Tasuku, Takuya</span>==== | |
| - | ====Wednesday, August 25 <span class="by">By:Ken, Tomo, Kazuya, Tasuku, Takuya<span>==== | + | |
=====Making culture and Master plate===== | =====Making culture and Master plate===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,281: | Line 1,271: | ||
| - | ====Thursday, August 26 <span class="by">By:Ken, Tomo, Kazuya, Tasuku, Takuya, Fumitaka</span> | + | ====Thursday, August 26 <span class="by">By:Ken, Tomo, Kazuya, Tasuku, Takuya, Fumitaka</span>==== |
| + | |||
=====Miniprep===== | =====Miniprep===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,342: | Line 1,333: | ||
{| class="experiments" | {| class="experiments" | ||
!Name||Colony | !Name||Colony | ||
| + | |- | ||
|R0011-rrS<sub>ΔTMD1</sub>-E0840||Many colonies | |R0011-rrS<sub>ΔTMD1</sub>-E0840||Many colonies | ||
|- | |- | ||
| Line 1,358: | Line 1,350: | ||
| - | ====Tuesday, August 31 <span class="by">By: Tomonori, Takuya Y., Kazuya, Tasuku, Takuya, Ken<span> | + | ====Tuesday, August 31 <span class="by">By: Tomonori, Takuya Y., Kazuya, Tasuku, Takuya, Ken</span>==== |
=====Miniprep===== | =====Miniprep===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,374: | Line 1,366: | ||
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| + | |- | ||
|J23105 (RPU0.3)||5.8 ng/µL | |J23105 (RPU0.3)||5.8 ng/µL | ||
|- | |- | ||
| Line 1,384: | Line 1,377: | ||
|R0011-rrS<sub>ΔTMD1</sub>-E0840||J23105 (RPU0.3) | |R0011-rrS<sub>ΔTMD1</sub>-E0840||J23105 (RPU0.3) | ||
|} | |} | ||
| - | |||
====Wednesday, September 1 <span class="by">By: Tomonori, Kazuya, Tasuku, Fumitaka, Ken</span>==== | ====Wednesday, September 1 <span class="by">By: Tomonori, Kazuya, Tasuku, Fumitaka, Ken</span>==== | ||
| Line 1,422: | Line 1,414: | ||
|pSB4K5||16.8 | |pSB4K5||16.8 | ||
|} | |} | ||
| - | ====Ligation and transformation==== | + | =====Ligation and transformation===== |
* Insert: SRRz-B0015 (1-1) | * Insert: SRRz-B0015 (1-1) | ||
* Vector: pSB4K5 | * Vector: pSB4K5 | ||
| - | ====Thursday, September 2 <span class="by">By: Tomonori, Tomo, Takuya, Ken<span>==== | + | ====Thursday, September 2 <span class="by">By: Tomonori, Tomo, Takuya, Ken</span>==== |
=====Making culture and Master plate===== | =====Making culture and Master plate===== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,450: | Line 1,442: | ||
*SRRz-B0015 (1-1) [pSB4K5] | *SRRz-B0015 (1-1) [pSB4K5] | ||
*SRRz-B0015 (1-2) [pSB4K5] | *SRRz-B0015 (1-2) [pSB4K5] | ||
| - | *R0011- | + | *ML |
| + | |||
| + | |||
| + | ====Monday, September 6 <span class="by">By: Wataru, Tomo, Kazuya, Ken</span>==== | ||
| + | =====Sequence Analysis===== | ||
| + | * R0011-rrS<sub>ΔTMD1</sub>-E0840 (A) | ||
| + | * R0011-rrS<sub>ΔTMD1</sub>-E0840 (B) | ||
| + | * SRRz-B0015 [pSB4K5] (A) | ||
| + | * SRRz-B0015 [pSB4K5] (B) | ||
| + | * rrS<sub>ΔTMD1</sub>-E0840 (1-1) | ||
| + | * rrS<sub>ΔTMD1</sub>-E0840 (1-2) | ||
| + | R0011-rrS<sub>ΔTMD1</sub>-E0840 (A), SRRz-B0015 [pSB4K5] (A) is correct. | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |SRRz||29.6 ng/µL | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840||70.2 | ||
| + | |- | ||
| + | |J23105 (RPU0.3)||75.3 | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1-1)||30.3 | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | * J23105 (RPU0.3): SpeI, PstI | ||
| + | * rrS<sub>ΔTMD1</sub>-E0840: XbaI, PstI | ||
| + | =====Gel Extraction===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |J23105 (RPU0.3) [SP]||20ng / 10µL | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840||100ng / 1µL | ||
| + | |} | ||
| + | =====Ligation===== | ||
| + | {| class="experiments" | ||
| + | !colspan="2"| Vector ||conspan="2"| Insert || Ligation High || Total || Incubation | ||
| + | |- | ||
| + | |J23105 (RPU0.3) [SP]||1 µL||rrS<sub>ΔTMD1</sub>-E0840 [XP]||1||2||4||30min | ||
| + | |- | ||
| + | |} | ||
| + | =====Transformation===== | ||
| + | {| class="experiments" | ||
| + | !Name||Competent Cell||Product of Ligation | ||
| + | |- | ||
| + | |J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840||50 µL||4|| | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | ====Tuesday, September 7 <span class="by">By: Wataru, Ken</span>==== | ||
| + | =====Insert Check===== | ||
| + | [[Image:KyotoExp100907-1.png|300px|right]] | ||
| + | We did colony PCR, and three colonies were inserted rrS<sub>ΔTMD1</sub>-E0840. So we succeeded in making J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840. | ||
| + | |||
| + | ====Thirsday, September 9 <span class="by">By: Wataru, Ken</span>==== | ||
| + | =====Culture===== | ||
| + | Culture pSB4K5 and SRRz<sub>Sam7</sub> | ||
| + | |||
| + | |||
| + | ====Friday, September 10 <span class="by">By: Wataru, Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |pSB4K5||48.8 | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>||33.4 | ||
| + | |} | ||
| + | =====Mutagenesis===== | ||
| + | We lost SRRz-B0015, so we decided to retry point mutation. | ||
| + | {| class="experiments" | ||
| + | !Name||MgS04||dNTP||10xBuffer||Template||KOD||MilliQ||Total | ||
| + | |- | ||
| + | |SRRz (1)||3||5||5||1.5||1||34||50 | ||
| + | |- | ||
| + | |SRRz (2)||3||5||5||1.5||1||34||50 | ||
| + | |- | ||
| + | |Control||3||5||5||1.5||1||34||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||1min|| | ||
| + | |- | ||
| + | |94℃||5s||rowspan="3"|25 cycles | ||
| + | |- | ||
| + | |55℃||30s | ||
| + | |- | ||
| + | |68℃||3min 40s | ||
| + | |- | ||
| + | |4℃||Forever|| | ||
| + | |} | ||
| + | After digestion by DpnI, Ligation and Transformation. | ||
| + | |||
| + | ====Sunday, September 12 <span class="by">By: Wataru</span>==== | ||
| + | =====Culture===== | ||
| + | Culture SRRz (1), (2), (3) from original plate. | ||
| + | |||
| + | |||
| + | ====Monday, September 13 <span class="by">By: Wataru, Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |SRRz (1)||61.3 ng/µL | ||
| + | |- | ||
| + | |SRRz (2)||59.3 | ||
| + | |- | ||
| + | |SRRz (3)||69.3 | ||
| + | |} | ||
| + | =====Sequence Analysis===== | ||
| + | * SRRz (1), (2), (3) | ||
| + | SRRz (1), (2), (3) are correct. | ||
| + | =====Restriction Digestion===== | ||
| + | * J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840: EcoRI, SpeI | ||
| + | * R0011: EcoRI, XbaI | ||
| + | =====Ligation===== | ||
| + | J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840 [ES] + R0011 [EX] | ||
| + | =====Transformation===== | ||
| + | |||
| + | |||
| + | ====Tuesday, September 14 <span class="by">By: Ken, Wataru</span>==== | ||
| + | =====Colony PCR===== | ||
| + | We did Colony PCR, and we succeeded in making J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840-R0011 | ||
| + | =====Sequence Analysis===== | ||
| + | From the product of Colony PCR. | ||
| + | * J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840-R0011 | ||
| + | J23105 (RPU0.3)-rrS<sub>ΔTMD1</sub>-E0840-R0011 is correct. | ||
| + | |||
| + | |||
| + | ====Tuesday, September 30 <span class="by">By: Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |LTC (1)||84.6 ng/µL | ||
| + | |- | ||
| + | |LTC (2)||97.6 | ||
| + | |- | ||
| + | |LTC (3)||127.4 | ||
| + | |- | ||
| + | |LTC (4)||85.4 | ||
| + | |- | ||
| + | |LTC (5)||70.0 | ||
| + | |} | ||
| + | |||
| + | |||
| + | ====Friday, October 1 <span class="by">By: Ken</span>==== | ||
| + | =====Sequence Analysis===== | ||
| + | Analyzed LTC 1-5. The sequencing on each sample was in success. The results of sequencing with VF2 were desirable, but, on the other, only one of those with VR was desirable, the others were found bad sequencing. We decided to use LTC 2. | ||
| + | |||
| + | |||
| + | ====Saturday, October 2 <span class="by">By: Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |SRRz-low||162.3 ng/µL | ||
| + | |- | ||
| + | |CTL||60.8 ng/µL | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | *LTC (EcoRI-SpeI) | ||
| + | *CTL (EcoRI-SpeI) | ||
| + | *SRRz low (EcoRI-XbaI) | ||
| + | |||
| + | ====Sunday, October 3 <span class="by">By: Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |SRRz-low||237.3 ng/µL | ||
| + | |- | ||
| + | |CTL||138.8 | ||
| + | |- | ||
| + | |LTC||441.2 | ||
| + | |- | ||
| + | |CT||252.1 | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||10xbuffer||100xbuffer||EcoRI||XbaI||SpeI||CIAP||Water||Total | ||
| + | |- | ||
| + | |SRRz-low||7||4||0.4||0.3||0.3||-||1||27||40 | ||
| + | |- | ||
| + | |CTL||10||4||0.4||0.3||-||0.3||-||25||40 | ||
| + | |- | ||
| + | |LTC||4||4||0.4||0.3||-||0.3||-||31||40 | ||
| + | |} | ||
| + | =====Concentration(after purification)===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |SRRz-low(E-X)||36.9 ng/µL | ||
| + | |- | ||
| + | |CTL(E-S)||25.0 | ||
| + | |- | ||
| + | |LTC(E-S)||64.3 | ||
| + | |} | ||
| + | =====Ligation and Transformation===== | ||
| + | Ligation and transformation of CTL-SRRz(CTLS) and LTC-SRRz(LTCS). | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |CTLS||Some colonies | ||
| + | |- | ||
| + | |LTCS||Some colonies | ||
| + | |} | ||
| + | |||
| + | ====Monday, October 4 <span class="by">By: Ken</span>==== | ||
| + | =====Making culture===== | ||
| + | Cultured CTLS and LTCS with LB-Kan(+/- IPTG). | ||
| + | |||
| + | ====Tuesday, October 5 <span class="by">By: Ken</span>==== | ||
| + | =====Results of Screening===== | ||
| + | The propagation wasn't observed on some samples on CTLS cultured with M9-Kan +IPTG. It meant that the construction of CTLS was in success. | ||
| + | However, the growth was observed on all of samples of LTCS. So, we decided to retry the construction of LTCS. | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |LT||387.1 ng/µL | ||
| + | |} | ||
| + | =====Making culture===== | ||
| + | Cultured CTLS. | ||
| + | |||
| + | ====Wednesday, October 6 <span class="by">By: Ken</span>==== | ||
| + | ===== ===== | ||
| + | |||
| + | |||
| + | ====Thursday, October 7 <span class="by">By: Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>||84.1 ng/µL | ||
| + | |- | ||
| + | |lac-SRRz||172.5 | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||10xbuffer||100xbuffer||EcoRI||PstI||Water||Total | ||
| + | |- | ||
| + | |LT||4||4||0.4||0.4||0.4||30.8||40 | ||
| + | |- | ||
| + | |CT||6||4||0.4||0.4||0.4||28.8||40 | ||
| + | |- | ||
| + | |CTL||11||4||0.4||0.4||0.4||23.8||40 | ||
| + | |- | ||
| + | |LTC||4||4||0.4||0.4||0.4||30.8||40 | ||
| + | |- | ||
| + | |SRRz||7||4||0.4||0.4||0.4||27.8||40 | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>||17||4||0.4||0.4||0.4||17.8||40 | ||
| + | |} | ||
| + | =====Concentration after purification===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |LT||107.9 ng/µL | ||
| + | |- | ||
| + | |CT||56.9 | ||
| + | |- | ||
| + | |CTL||37.1 | ||
| + | |- | ||
| + | |LTC||101.9 | ||
| + | |- | ||
| + | |SRRz||55.7 | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>||102.9 | ||
| + | |} | ||
| + | |||
| + | ====Friday, October 8 <span class="by">By: Ken</span>==== | ||
| + | =====Screening===== | ||
| + | Screened lac-SRRz 1-7(LB-Tc with IPTG 1.5mM). Except for sample 4, the propagation could be observed. So, we decided to use sample 4. | ||
| + | =====Restriction Digestion of pSB1C3===== | ||
| + | {| class="experiments" | ||
| + | !Template||10xbuffer||100xbuffer||EcoRI||PstI||Water||Total | ||
| + | |- | ||
| + | |5||4||0.4||0.4||0.4||28.4 | ||
| + | |} | ||
| + | =====Concentration after purification===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |pSB1C3(E-P)||16.1 ng/µL | ||
| + | |} | ||
| + | =====Ligation and Transformation===== | ||
| + | Inserted LTC, CTL, T, SRRz, LT or CT to pSB1C3. | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |LTC||Some colonies | ||
| + | |- | ||
| + | |CTL||No colony | ||
| + | |- | ||
| + | |T||Some colonies | ||
| + | |- | ||
| + | |SRRz||Some colonies | ||
| + | |- | ||
| + | |LT||Some colonies | ||
| + | |- | ||
| + | |CT||Some colonies | ||
| + | |} | ||
| + | |||
| + | ====Saturday, October 9 <span class="by">By: Ken</span>==== | ||
| + | =====Making culture===== | ||
| + | Cultured LTC, T, SRRz, LT, and CT with LB (Cp/Amp+). | ||
| + | =====Restriction digestion of LTC and SRRz-low===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||10xbuffer||100xbuffer||EcoRI||SpeI||Water||Total | ||
| + | |- | ||
| + | |LTC||3.5||4||0.4||0.6||0.6||30.9||40 | ||
| + | |- | ||
| + | |SRRz-low||4||4||0.4||0.6||0.6||30.4||40 | ||
| + | |} | ||
| + | =====Ligation and Transformation of LTCS===== | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |LTCS||Many colonies | ||
| + | |- | ||
| + | |control||few colonies | ||
| + | |} | ||
| + | Used Top 10. | ||
| + | |||
| + | ====Sunday, October 10 <span class="by">By: Ken</span>==== | ||
| + | =====Screening===== | ||
| + | Screened LTC, T, SRRz, LT, and CT. Except for LT1, the propagation could not be observed on each sample with LB (Amp+). | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |LTC1||210.6 ng/µL | ||
| + | |- | ||
| + | |T2||193.2 | ||
| + | |- | ||
| + | |SRRz3||208.9 | ||
| + | |- | ||
| + | |LT2||195.7 | ||
| + | |- | ||
| + | |CT1||219.9 | ||
| + | |- | ||
| + | |CTLS4-2||231.0 | ||
| + | |- | ||
| + | |LTCS1||50.6 | ||
| + | |} | ||
| + | =====Restriction Digestion of LTC, T, SRRz, LT, CT, and CTLS===== | ||
| + | {| class="experiments" | ||
| + | !Template||10xBuffer||100xBuffer||EcoRI||PstI||MilliQ||Incubation | ||
| + | |- | ||
| + | |2 µL||1||0.1||0.2||0.2||6.5||60min | ||
| + | |} | ||
| + | =====Electrophoreis===== | ||
| + | [[Image:KyotoExp101010-1.png|300px|right]] | ||
| + | * Lane 1, 8: Lambda, 100bp Marker | ||
| + | * Lane 2-7: LTC, T, SRRz, LT, CT, CTLS | ||
| + | We could observe that the each part was correctly inserted to the pSB1C3. | ||
| + | |||
| + | =====Retry - Transformation of CTL (pSB1C3) and LTCS===== | ||
| + | Used KRX. | ||
| + | =====Deletion PCR of T2===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||2xBuffer||dNTPs||MgSO4||Primer1||Primer2||Primer3||KOD-FX||MilliQ||Total | ||
| + | |- | ||
| + | |GFP-Deletion1, 2||0.25 µL||25||10||3||1.5||-||1.5||1||8.75||50 | ||
| + | |- | ||
| + | |GFP-DT-Deletion1, 2||0.25 µL||25||10||3||-||1.5||1.5||1||8.75||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |98℃||10s||rowspan="3"|30 cycles | ||
| + | |- | ||
| + | |59℃||30s | ||
| + | |- | ||
| + | |68℃||3.5min | ||
| + | |- | ||
| + | |4℃||Hold|| | ||
| + | |} | ||
| + | Primer: | ||
| + | # ccaggcatcaaataaaacgaaaggctc | ||
| + | # tactagtagcggccgctgc | ||
| + | # ctctagtattattgatttctaccatcttctactcc | ||
| + | |||
| + | |||
| + | |||
| + | ====Monday, October 11 <span class="by">By: Ken</span>==== | ||
| + | =====Restriction Digestion, Electrophoresis, Ligation and Transformation of PCR products===== | ||
| + | {| class="experiments" | ||
| + | !Template||DpnI||Incubation | ||
| + | |- | ||
| + | |25 µL||1||60min | ||
| + | |} | ||
| + | |||
| + | [[Image:KyotoExp101011-1.png|300px|right]] | ||
| + | {| class="electrophoresis" | ||
| + | !Lane||Name | ||
| + | |- | ||
| + | |1,6||Lambda, 100bp Marker | ||
| + | |- | ||
| + | |2||GFP-Deletion1 | ||
| + | |- | ||
| + | |3||GFP-Deletion2 | ||
| + | |- | ||
| + | |4||GFP-DT-Deletion1 | ||
| + | |- | ||
| + | |5||GFP-DT-Deletion2 | ||
| + | |} | ||
| + | Deletion PCR was well performed. | ||
| + | |||
| + | {| class="experiments" | ||
| + | !Template||Ligation High||T4 Kinase||Incubation | ||
| + | |- | ||
| + | |9 µL||5||1||90min | ||
| + | |} | ||
| + | |||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>-dT||Many colonies | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>||Many colonies | ||
| + | |} | ||
| + | *Competent Cell: TOP10 | ||
| + | |||
| + | =====Sequence===== | ||
| + | Sequenced LTC1, T2, SRRz3, LT2, CT1, CTLS4-2 and LTCS1. | ||
| + | {| class="experiments" | ||
| + | !Template||5xBuffer||Primer||Big Dye||MilliQ||Total | ||
| + | |- | ||
| + | |(200ng)||2 µL||1||0.5||6.5||10 | ||
| + | |} | ||
| + | Primer: | ||
| + | # VF2 | ||
| + | # VR | ||
| + | # aggtgatgcaacatacggaaaacttacc | ||
| + | # tgctgggattacacatggcatggatg | ||
| + | # ttcctcgatatgctggcgtggtc | ||
| + | Used Primer 1 or 2 to each sample, and Primer 3, 4, or 5 only to CTLS4-2 and LTCS1. | ||
| + | {| class="experiments" | ||
| + | |96℃||1min|| | ||
| + | |- | ||
| + | |96℃||10s||rowspan="3"|40 cycles | ||
| + | |- | ||
| + | |50℃||5s | ||
| + | |- | ||
| + | |60℃||2min 5s | ||
| + | |- | ||
| + | |4℃||Hold|| | ||
| + | |} | ||
| + | Except for CTLS4-2 and LTCS1 with Primer2, the sequencing of each sample were well performed. And LCT1, T2, LT2, and CT1 were confirmed that the sequence were correct. There was, however, deletion of some base pairs on lactose promoter [R0011]. of CTLS402. | ||
| + | |||
| + | * Result: AATTGTGAGCGGATAACAAGATACTGAGCACA | ||
| + | * BBa_R0011: AATTGTGAGCGGATAACAATTGACATTGTGAGCGGATAACAAGATACTGAGCACA | ||
| + | |||
| + | There is repeated sequence on lactose promoter and the one was deleted. So, we supposed that the homologous recombination was occureed. From this result, we could say that the function check of CTLS was failed because SRRz gene wasn't induced by IPTG. | ||
| + | =====Making culture of LTCS and CTL===== | ||
| + | |||
| + | ====Tuesday, October 12 <span class="by">By: Ken</span>==== | ||
| + | =====Screening of CTL===== | ||
| + | The propagation could not be observed with LB-Amp and could be observed with LB-Cp. It meant that the part, CTL, was correctly inserted to the plasmid, pSB1C3. | ||
| + | =====Making culture===== | ||
| + | Cultured two samples on each part: S<sub>ΔTMD1</sub>-dT and S<sub>ΔTMD1</sub>. Named T1,3,4 and 6. | ||
| + | |||
| + | ====Wednesday, October 13 <span class="by">By: Ken</span>==== | ||
| + | ===== ===== | ||
| + | |||
| + | |||
| + | ====Thursday, October 14 <span class="by">By: Ken</span>==== | ||
| + | =====Retry of the construction of CTLS===== | ||
| + | |||
| + | ====Friday, October 15 <span class="by">By: Ken</span>==== | ||
| + | =====Screening of CTLS===== | ||
| + | Cultured CTLS with M9+IPTG. And the propagation couldn't be observed on one sample. | ||
| + | We decided to use this sample. | ||
| + | =====Sequence Analysis===== | ||
| + | We did the sequencing on ML, MS, CTLS, T4 and CTL. The sequencing of T4 and CTL were in success and the others were failed. The results of CTL was desirable, but that of T4 was bad sequence and there was a mutation on PstI site. Including the last time sequencing, we could say that the deletion of GFP-terminator from S<sub>ΔTMD1</sub>-GFP was failed. So, we decided to retry the deletion PCR. | ||
| + | |||
| + | |||
| + | |||
| + | ====Sunday, October 17 <span class="by">By: Ken</span>==== | ||
| + | =====Retry of deletion PCR of T2===== | ||
| + | We used PCR to delete GFP and terminator[E0840] from S<sub>ΔTMD1</sub>-E0840. | ||
| + | {| class="experiments" | ||
| + | !Name||Template||2xBuffer||dNTPs||Primer(fwd)||Primer(rev)||KOD-FX||MilliQ||Total | ||
| + | |- | ||
| + | |GFP-DT-Deletion1, 2||0.25 µL||25||10||1.5||1.5||1||10.75||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |98℃||10s||rowspan="2"|30 cycles | ||
| + | |- | ||
| + | |68℃||3.5min | ||
| + | |- | ||
| + | |4℃||Hold|| | ||
| + | |} | ||
| + | After the PCR, digestion by DpnI(37C/60min). | ||
| + | |||
| + | =====Electrophoresis===== | ||
| + | [[Image:KyotoExp101017-1.png|300px|right]] | ||
| + | *Lane 1,2,5,6: Lambda, 100bp Maker | ||
| + | *Lane 3,4 PCR products[T'1,T'2] | ||
| + | PCR was in success. | ||
| + | =====Ligation===== | ||
| + | Ligation PCR products. | ||
| + | |||
| + | ====Monday, October 18 <span class="by">By: Ken</span>==== | ||
| + | =====Transformation===== | ||
| + | Transformed T'1 and T'2. | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |T'1||Some colonies | ||
| + | |- | ||
| + | |T'2||Some colonies | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | ====Thursday, October 21 <span class="by">By: Ken</span>==== | ||
| + | =====Making Culture===== | ||
| + | Cultured two samples on each plate, T'1 and T'2. Named dele1-4. And also cultured lac-SRRz. | ||
| + | |||
| + | ====Friday, Ocotber 22 <span class="by">By: Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |dele1||117.9 ng/µL | ||
| + | |- | ||
| + | |dele2||130.8 | ||
| + | |- | ||
| + | |dele3||108.7 | ||
| + | |- | ||
| + | |dele4||176.0 | ||
| + | |- | ||
| + | |lac-SRRz||67.2 | ||
| + | |} | ||
| + | |||
| + | ====Saturday, October 23 <span class="by">By: Ken</span>==== | ||
| + | =====Sequence Analysis===== | ||
| + | We used VF2 on dele1-4 and lac-SRRz and VR on lac-SRRz. | ||
| + | The sequencing of lac-SRRz with VR was failed, the others were in success. | ||
| + | The results were desirable on dele1,3,4 and lac-SRRz. | ||
| + | |||
| + | =====Deletion PCR of lac-SRRz===== | ||
| + | We used deletion PCR to get lac-S<sub>ΔTMD1</sub>RRz. | ||
| + | {| class="experiments" | ||
| + | !Name||Template||2xBuffer||dNTPs||Primer1||Primer2||KOD||MilliQ||Total | ||
| + | |- | ||
| + | |lac-S<sub>ΔTMD1</sub>RRz (1)||0.8||25||10||1.5||1.5||1||10.2||50 | ||
| + | |- | ||
| + | |lac-S<sub>ΔTMD1</sub>RRz (2)||0.8||25||10||1.5||1.5||1||10.2||50 | ||
| + | |- | ||
| + | |control||0.8||25||10||1.5||1.5||0||11.2||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |98℃||10s||rowspan="2"|30 cycles | ||
| + | |- | ||
| + | |68℃||5min | ||
| + | |- | ||
| + | |4℃||Hold|| | ||
| + | |} | ||
| + | After the PCR, done restriction digestion by DpnI(37℃/60min). | ||
| + | |||
| + | =====Electrophoresis===== | ||
| + | [[Image:KyotoExp101023-1.png|300px|right]] | ||
| + | *Lane 1,8,2,7: Lambda, 100bp Maker | ||
| + | *Lane3,4: lac-S<sub>ΔTMD1</sub>RRz (1,2) | ||
| + | *Lane5: control | ||
| + | |||
| + | =====Ligation and Transformation===== | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |lac-S<sub>ΔTMD1</sub>RRz(1)||some colonies | ||
| + | |- | ||
| + | |lac-S<sub>ΔTMD1</sub>RRz(2)||some colonies | ||
| + | |- | ||
| + | |control||no colony | ||
| + | |} | ||
| + | Used KRX. | ||
| + | |||
| + | ====Sunday, October 24 <span class="by">By: Ken</span>==== | ||
| + | =====Making Culture===== | ||
| + | Cultured CTLS, Lysisbox and three samples of lac-S<sub>ΔTMD1</sub>RRz with LB-Tc(IPTG 0 or 1mM). | ||
| + | |||
| + | ====Monday, October 25 <span class="by">By: Ken</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |CTLS||62.9 ng/µL | ||
| + | |- | ||
| + | |Lysisbox||31.9 | ||
| + | |- | ||
| + | |lac-S<sub>ΔTMD1</sub>RRz 1||140.9 | ||
| + | |- | ||
| + | |lac-S<sub>ΔTMD1</sub>RRz 2||71.6 | ||
| + | |} | ||
| + | The propagation was not observed on lac-S<sub>ΔTMD1</sub>RRz 3 with LB (IPTG 1mM). | ||
| + | It meant this sample might be lac-SRRz. We decided not to use this. | ||
| + | =====PCR===== | ||
| + | We used PCR on CTLS and Lysisbox to change its own constitutive promoter to another. | ||
| + | {| class="experiments" | ||
| + | !Name||Template||2xBuffer||dNTPs||Primer1||Primer2||Primer3||Primer4||KOD-FX||MilliQ||Total | ||
| + | |- | ||
| + | |C'TLS(1)||0.8 µL||25||10||1.5||1.5||-||-||1||10.2||50 | ||
| + | |- | ||
| + | |C'TLS(2)||0.8 µL||25||10||1.5||1.5||-||-||1||10.2||50 | ||
| + | |- | ||
| + | |Lysisbox'(1)||1.5 µL||25||10||-||-||1.5||1.5||1||9.5||50 | ||
| + | |- | ||
| + | |Lysisbox'(2)||1.5 µL||25||10||-||-||1.5||1.5||1||9.5||50 | ||
| + | |} | ||
| + | |||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |98℃||10s||rowspan="2"|30 cycles | ||
| + | |- | ||
| + | |68℃||6min 20s | ||
| + | |- | ||
| + | |4℃||Hold|| | ||
| + | |} | ||
| + | Primer | ||
| + | # aggtacagtgctagctactagaggagc | ||
| + | # aggactgagctagccgtcaactc | ||
| + | # aggtattatgctagctactagaggagc | ||
| + | # aggactgagctagctgtaaactctag | ||
| + | |||
| + | =====Electrophoresis===== | ||
| + | [[Image:KyotoExp101025-1.png|300px|right]] | ||
| + | *Lane 1,8,2,7: Lambda, 100bp Maker | ||
| + | *Lane 3,4: C'TLS(1,2) | ||
| + | *Lane 5,6: Lysisbox'(1,2) | ||
| + | Two bands were observed on lane 5 and 6. We decided to do gel extraction. | ||
| + | |||
| + | =====Ligation===== | ||
| + | We did ligation of C'TLS(1,2). | ||
| + | |||
| + | ====Tuesday, October 26 <span class="by">By: Ken</span>==== | ||
| + | =====Gel Extraction of Lysisbox'===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |Lysisbox'(1)||33.6 ng/µL | ||
| + | |- | ||
| + | |Lysisbox'(2)||43.4 ng/µL | ||
| + | |} | ||
| + | =====Ligation===== | ||
| + | We did ligation of Lysisbox'(1,2). | ||
| + | =====Transformaiton===== | ||
| + | Transformed C'TLS(1,2) and Lysisbox'(1,2). | ||
---- | ---- | ||
Latest revision as of 02:17, 28 October 2010
Notebook1
Construction
Tuesday, July 20 By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto
Transformation
| Name | Well | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| J23100 | 1-18-C | 1 µL | 20 | 21 | LB (Amp+) | At 37℃, 7/20 20:50 - 7/21 17:00 | ○ |
| J23105 | 1-18-M | 1 | 20 | 21 | ○ | ||
| J23116 | 1-20-M | 1 | 20 | 21 | ○ | ||
| R0011 | 1-6-G | 1 | 20 | 21 | ○ | ||
| E0840 | 1-12-O | 1 | 20 | 21 | ○ | ||
| J06702 | 2-8-E | 1 | 20 | 21 | ○ | ||
| pSB4K5 | 1-5-G | 1 | 20 | 21 | × | ||
| B0015 | 1-23-L | 1 | 20 | 21 | LB (Kan+) | × |
A vector of pSB4K5 is Kanamycin-resistance, however, we plated it to LB plate (Amp+). And We didn't pre-culture B0015 despite its vector is Kanamycin-resistance. So, it was predicted that we will fail the transformation of pSB4K5 and B0015.
Wednesday, July 21 By: Wataru, Ken, Makoto, Takuya Y.
Culture at 37℃ from 07/21 20:50 to 07/22 17:00 and Making Master Plate
Transformation
| Name | Well | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| pSB4K5 | 1-5-G | 1 µL | 20 | 21 | LB (Kan+) | At 37℃, 7/21 20:50 - 7/22 16:30 | ○ |
| B0015 | 1-23-L | 1 | 20 | 21 | ○ |
PCR for SRRz and S
| No. | Water | MgSO4 | dNTPs | 10xBuffer | Template DNA | Primer Fwd. | Primer Rev. (SRRz) | Primer Rev. (S) | KOD Plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 µL | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 2 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 3 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 4 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 5 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 6 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 7 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 8 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Thursday, July 22 By: Wataru
Electrophoresis (40min) of the PCR Products
- Sample: 1, 2, 5, 6 = SRRz, 3, 4, 7, 8 = S
- Marker: 100bp, 1kb, 1kb, 100bp.
Miniprep
| Name | Concentration |
|---|---|
| J23100 | 18.5 (ng/µL) |
| J23105 | 12.5 |
| J23116 | 14.6 |
| R0011 | 8.6 |
| E0840 | 12.1 |
| J06702 | 14.7 |
The concentration of all samples was very week. Probably our shaking incubation was week.
Culture from 07/22 17:00 to 07/23 10:00 and Making Master Plates of pSB4K5 and B0015
Friday, July 23 By: Wataru, Tomo, Makoto
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 79.2 (ng/µL) |
| B0015 | - |
We lost B0015 by our mistake. The concentration of pSB4K5 is high, so this condition of shaking incubation is moderate.
PCR Purification
| No. | Name | Concentration | New Name |
|---|---|---|---|
| 1 | SRRz | 18.6 ng/µL | - |
| 3 | S | 77.6 | SSam7(1) |
| 5 | SRRz | 33.6 | - |
| 7 | S | 65.4 | SSam7(2) |
The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR.
PCR for SRRz
| No. | Water | MgSO4 | dNTPs | 10xBuffer | Template DNA | Primer Fwd. (SRRz) | Primer Rev. (SRRz) | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 µL | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 2 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 3 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 4 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 5 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 6 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Restriction Digestion and Electrophoresis (35min) to check function of our Restriction Enzyme
| No. | Name | Sample | 10xBuffer | BSA | Enzyme | MilliQ | Total | Incubation | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | J06702 | 5 µL | 1 | 0.1 | EcoRI | 0.1 | 3.6 | 10 | At 37℃ 7/23 18:00 - 7/23 18:30 |
| 2 | J06702 | 5 | 1 | 0.1 | XbaI | 0.1 | 3.6 | 10 | |
| 3 | J06702 | 5 | 1 | 0.1 | SpeI | 0.1 | 3.6 | 10 | |
| 4 | J06702 | 5 | 1 | 0.1 | PstI | 0.1 | 3.6 | 10 | |
| 5 | J06702 | 5 | 1 | 0.1 | - | 3.7 | 10 | ||
Marker: 1kb. Comparison to No. 5 (control, circular DNA), the bands of No. 1, 2, 3, and 4 was shifted. The DNA of them was linearized by Restriction enzymes. So, our restriction enzymes work correctly.
Restriction Digestion and Ligation to insert S gene to E0840
| Name | Sample | 10xBuffer | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|
| SSam7(1) | 11 µL | 5 | EcoRI | 0.2 | SpeI | 0.2 | 33.6 | 50 | At 37℃ for 2h |
| SSam7(2) | 11 | 5 | EcoRI | 0.2 | SpeI | 0.2 | 33.6 | 50 | |
| E0840 | 45 | 5 | EcoRI | 0.2 | XbaI | 0.2 | 0 | 50 | |
After PCR Purification, evaporated them and diluted 3µL.
| Name | Vector | Insert | Ligation High | Total | ||
|---|---|---|---|---|---|---|
| SSam7(1)-E0840 | E0840 | 0.5µL | SSam7(1) | 0.5 | 1 | 2 |
| SSam7(2)-E0840 | E0840 | 0.5 | SSam7(2) | 0.5 | 1 | 2 |
Monday, July 26 By: Wataru, Tomonori, Makoto
Electrophoresis of PCR Products
| No. | Name | Length(bp) | Result |
|---|---|---|---|
| 1 | SRRz | 1386 | |
| 2 | SRRz | 1386 | |
| 3 | SRRz | 1386 | |
| 4 | SRRz | 1386 | |
| 5 | SRRz | 1386 | |
| 6 | SRRz | 1386 |
Marker: 1kb. At the condition 4 (4.5µL MgSO4) and 6 (6µL MgSO4), SRRz is amplified very much. So we decided to use them.
PCR Purification
| No. | Name | Concentration | New Name |
|---|---|---|---|
| 4 | SRRZ | 51.6 ng/µL | SRRzSam7(1) |
| 5 | SRRZ | 59.3 | |
| 6 | SRRZ | 59.6 | SRRzSam7(2) |
Transformation
| Name | Well | Sample | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| E0240 | 1-12-M | 1 µL | 20 | 21 | LB (Amp+) | At 37℃ 7/26 - 7/27 | × |
| I20260 | 2-17-F | 1 | 20 | 21 | LB (Kan+) | × | |
| J04450 | 1-5-E | 1 | 20 | 21 | × |
Culture of pSB4K5, E0840, and B0015
Tuesday, July 27 By: Wataru, Tomo, Kazuya, Ken, Naoi
Colony PCR of SSam7-E0840 (Electrophoresis for 35min)
| No. | Name | Length | Result |
|---|---|---|---|
| 1 | SSam7(1)-E0840 | 1522 | ○ |
| 2 | SSam7(1)-E0840 | 1522 | × |
| 3 | SSam7(1)-E0840 | 1522 | ○ |
| 4 | SSam7(1)-E0840 | 1522 | × |
| 5 | SSam7(1)-E0840 | 1522 | ○ |
| 6 | SSam7(1)-E0840 | 1522 | ◎ (Use as SSam7(1)-E0840) |
| 7 | SSam7(2)-E0840 | 1522 | × |
| 8 | SSam7(2)-E0840 | 1522 | × |
| 9 | SSam7(2)-E0840 | 1522 | × |
| 10 | SSam7(2)-E0840 | 1522 | × |
| 11 | SSam7(2)-E0840 | 1522 | ◎ (Use as SSam7(2)-E0840) |
| 12 | SSam7(2)-E0840 | 1522 | ○ |
| 13 | SSam7(2)-E0840 | 1522 | ○ |
| + | E0840 | 1116 | |
| - | None |
Marker: 1kb, 100bp
Miniprep
| Name | Concentration |
|---|---|
| R0011 | 26.9 ng/µL |
| B0015 | 120.0 |
| E0840 | 120.1 |
Restriction Digestion
| Name | Sample | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| B0015 | 30 µL | 5 | 0.5 | EcoRI | 0.4 | XbaI | 0.3 | 13.7 | 50 | At 37℃ 16:45 - 18:00 |
| SRRzSam7(1) | 40 | 5 | 0.5 | EcoRI | 0.4 | SpeI | 0.4 | 3.8 | 50 | |
| SRRzSam7(2) | 40 | 5 | 0.5 | EcoRI | 0.4 | SpeI | 0.4 | 3.8 | 50 | |
Ligation
Transformation
| Name | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| SRRzSam7(1)-B0015 | ○ | |||||
| SRRzSam7(2)-B0015 | ○ |
Wednesday, July 28 By:
Miniprep
| Name | Concentration |
|---|---|
| SSam7(1)-E0840 | 95.5 ng/µL |
| SSam7(2)-E0840 | 98.6 |
Diluted SSam7(1)-E0840 and SSam7(2)-E0840 20 times with water, and used as template DNA.
Deletion PCR to delete a functional domain of S gene
| Water | MgSO4 | dNTPs | 10xBuffer | Primer Fwd. | Primer Rev. | Template (1) | Template (2) | KOD Plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 28 µL | 3 | 5 | 5 | 1.5 | 1.5 | 5 | - | 1 | 50 |
| SSam7,ΔTMD1(1)-E0840 (2) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | - | 1 | 50 |
| SSam7,ΔTMD1(2)-E0840 (1) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | - | 5 | 1 | 50 |
| SSam7,ΔTMD1(2)-E0840 (2) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | - | 5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 35 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Restriction Digestion to check the function of DpnI
| Name | Sample | fast digestion buffer | DpnI | MilliQ | Total |
|---|---|---|---|---|---|
| SSam7(1)-E0840 | 3 µL | 1 | 0.1 | 5.8 | 10 |
| SSam7(2)-E0840 | 3 | 1 | 0.1 | 5.8 | 10 |
Electrophoresis for 35min
| No. | Name | Length | Result |
|---|---|---|---|
| 1 | Not digested SSam7(1)-E0840 | 3363bp | |
| 2 | Not digested SSam7(2)-E0840 | 3363 | |
| 3 | Digested SSam7(1)-E0840 | 1021, 933, 402, 341, 258, 105, ... | |
| 4 | Digested SSam7(2)-E0840 | 1021, 933, 402, 341, 258, 105, ... |
Marker: 1kb, 100bp DpnI works correctly.
Thursday, July 29 By:
Restriction Digestion
| Name | Sample volume | Fastdigestion Buffer | Enzyme 1 | MilliQ | Total | Incubation | |
|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 50 µL | 6 | DpnI | 0.2 | 3.8 | 60 | 07/29 09:40 - 07/29 11:00 |
| SSam7,ΔTMD1(2)-E0840 (1) | 50 | 6 | DpnI | 0.2 | 3.8 | 60 | |
Ligation and Phosphorylation
| Name | Sample | MilliQ | Ligation High | T4 Kinase | Total | Incubation |
|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 2 µL | 7 | 5 | 1 | 15 | 07/29 11:30 ~ 07/29 13:00 |
| SSam7,ΔTMD1(2)-E0840 (1) | 2 | 7 | 5 | 1 | 15 |
Transformation
| Name | Sample Volume | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 3 µL | 30 | 33 | LB (Amp+) | 07/29 ~ 07/30 | ○ |
| SSam7,ΔTMD1(2)-E0840 (1) | 3 | 30 | 33 | ○ |
Monday, August 2 By: Wataru, Ken
Miniprep
| Name | Concentration |
|---|---|
| SSam7,ΔTMD1-E0840 (1) | 52.7 ng/µL |
| SSam7,ΔTMD1-E0840 (2) | 54.4 |
| SSam7,ΔTMD1-E0840 (3) | 89.5 |
| pSB4K5 | 50.7 |
| R0011 | 18.6 |
PCR of E0240
E0240 is very important parts to measure RPU of promoters in iGEM. However, we failed to transfect it to E.coli from parts kit of iGEM. So we decided to amplify this parts by PCR.
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template E0240 | KOD Pllus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| E0240(1) | 28 µL | 3 | 5 | 5 | 1.5 | 1.5 | 5 | 1 | 50 |
| E0240(2) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 35 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Electrophoresis
PCR Purification
| Name | Concentration |
|---|---|
| E0240(1) | 42.6 ng/µL |
| E0240(2) | 55.3 |
Restriction Digestion for inserting E0240 to pSB4K5 by 3A assembly
| Name | Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| E0240(1) [XP] | 30 µL | 5 | 0.5 | XbaI | 0.2 | PstI | 0.2 | 14.1 | 50 |
| E0240(2) [XP] | 30 | 5 | 0.5 | XbaI | 0.2 | PstI | 0.2 | 14.1 | 50 |
PCR Purification
| Name | Concentration | Volume |
|---|---|---|
| E0240(1) [XP] | 21.8 ng/µL | 40 µL |
| E0240(2) [XP] | 32.4 | 45 |
Stored at -20℃.
Error PCR
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template (1) | Template (2) | Template (3) | KOD Pllus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | 32 µL | 3 | 5 | 5 | 1.5 | 1.5 | 1 | - | - | 1 | 50 |
| SSam7,ΔTMD1-E0840 (2) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | 1 | - | 1 | 50 |
| SSam7,ΔTMD1-E0840 (3) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | - | 1 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 20 cycles |
| 68℃ | 4min | |
| 4℃ | forever |
Restriction Digestion of SSam7,ΔTMD1-E0840 by DpnI
Transformation
| Name | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | 2 µL | 20 | 22 | - | - | ○ |
| SSam7,ΔTMD1-E0840 (2) | 2 | 20 | 22 | × | ||
| SSam7,ΔTMD1-E0840 (3) | 2 | 20 | 22 | ○ |
Tuesday, August 3 By:
Culture
Picked two colonies from SSam7,ΔTMD1-E0840 (1), and SSam7,ΔTMD1-E0840 (3), and cultured at 37℃ from 08/03 to 08/04.
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 60.7 ng/µL |
| R0011 | 26.8 |
Restriction Digestion
| Name | Sample | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| R0011 [ES] | 50 µL | 6 | 0.6 | EcoRI | 0.2 | SpeI | 0.2 | 3 | 60 |
| pSB4K5 [EP] | 50 | 6 | 0.6 | EcoRI | 0.2 | PstI | 0.2 | 3 | 60 |
| E0240(1) [XP] | 50 | 6 | 0.6 | XbaI | 0.2 | PstI | 0.2 | 3 | 60 |
| E0240(2) [XP] | 50 | 6 | 0.6 | XbaI | 0.2 | PstI | 0.2 | 3 | 60 |
PCR Purification
| Name | Concentration |
|---|---|
| pSB4K5 [EP] | 39.5 ng/µL |
| E0240(1) [XP] | 21.8 |
| E0240(2) [XP] | 32.4 |
pSB4K5 [EP] is concentrated 10µL and E0240(1) [XP], E0240(2) [XP] are concentrated 1µL.
Ethanol Precipitation
After ethanol precipitation, we diluted pSB4K5 by 2µL MilliQ
Ligation
| Name | Vector | Insert 1 | Insert 2 | Ligation High | T4 Kinase | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|
| ML (1) | pSB4K5 [EP] | 1 | R0011 [ES] | 1 | E0240(1) [XP] | 1 | 3 | 15 | 17:30 - 20:20 |
| ML (2) | pSB4K5 [EP] | 1 | R0011 [ES] | 1 | E0240(2) [XP] | 1 | 3 | 15 | |
PCR of I20260
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template I20260 | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| I20260 (1) | 32µL | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| I20260 (2) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
PCR Purification
| Name | Concentration |
|---|---|
| I20260 | 40.6 ng/µL |
Restriction Digestion
| Name | Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| I20260 [EP] | 45 µL | 6 | 0.6 | EcoRI | 0.2 | PstI | 0.2 | 8 | 60 |
PCR Purification
| Name | Concentration | Volume |
|---|---|---|
| I20260 [EP] | 74.1 ng/µL | 30 |
I20260 [EP] is concentrated at 7µL
Ligation
| Vector | Insert | Ligation High | Total | Incubation | |||
|---|---|---|---|---|---|---|---|
| MS | pSB4K5 [EP] | 1 | I20260 [EP] | 1 | 2 | 4 | 20:00-20:30 |
Transformation
| Name | Sample | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| ML (1) | 1 µL | 20 | 21 | LB (Kan+) | 08/03-08/04 | ○ |
| ML (2) | 1 | 20 | 21 | ○ | ||
| MS | 1 | 20 | 21 | ○ |
Thursday, August 5 By:
Culture and Master Plates
pSB4K5 is inserted RFP generator. We didn't distinguish this inserted parts from low copy plasmid backbone, so self-ligated colony is red. So, white colony is correctly inserted parts.
However, white colonies and green colonies are observed in ML (1) and ML (2) plate. We cultured both white and green colonies.
In MS, Many of colonies are red, but green colonies are observed. We cultured green colonies.
| Name | Color | Incubation |
|---|---|---|
| ML (1-1) | Green Colony | 8/5-8/6 |
| ML (1-2) | Green Colony | |
| ML (1-3) | White Colony | |
| ML (1-4) | White Colony | |
| ML (2-1) | Green Colony | |
| ML (2-2) | White Colony | |
| ML (2-3) | White Colony | |
| ML (2-4) | White Colony | |
| MS (1) | Green Colony | |
| MS (2) | Green Colony | |
| MS (3) | Green Colony |
Sequence
| Name | Concentration |
|---|---|
| SΔTMD1-E0840(1) A | 28.9 ng/µL |
| SΔTMD1-E0840(1) B | 25.3 |
| SΔTMD1-E0840(3) A | 26.6 |
| SΔTMD1-E0840(3) B | 24.0 |
As a result, deletion is succeeded, however, point mutation is failed. It is because DpnI is too little to digest all of template DNA.
Friday, August 6
Miniprep
| Name |
|---|
| ML (1-1) |
| ML (1-2) |
| ML (1-3) |
| ML (1-4) |
| ML (2-1) |
| ML (2-2) |
| ML (2-3) |
| ML (2-4) |
| MS (1) |
| MS (2) |
| MS (3) |
Restriction Digestion
| Name | Sample | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| ML (1-1) [EP] | 50 µL | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (1-2) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (1-3) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (1-4) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (2-1) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (2-2) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (2-3) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| ML (2-4) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| MS (1) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| MS (2) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
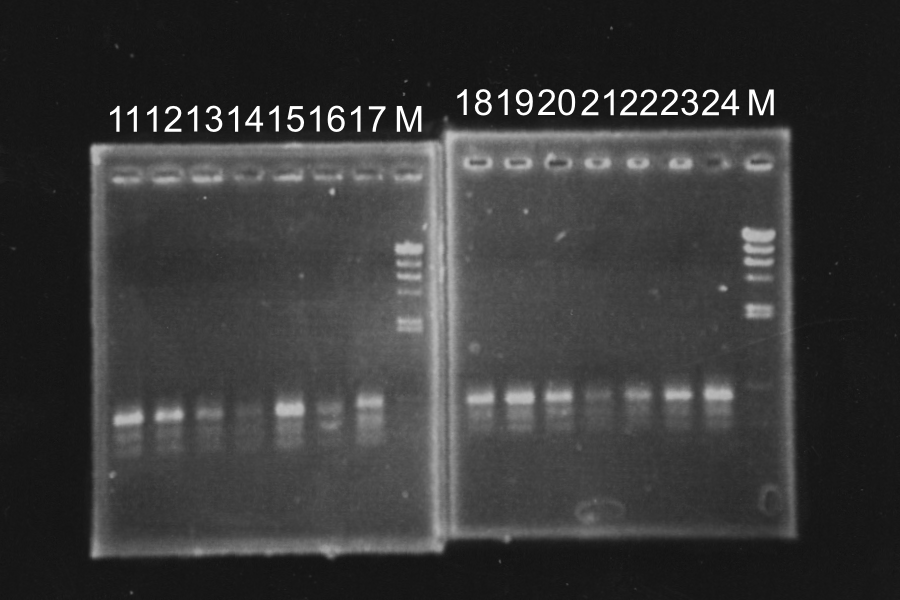
| MS (3) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
Electrophoresis
| No. | Name | Length | Results |
|---|---|---|---|
| 1 | MS (1) [EP] | 960, 4339 | |
| 2 | MS (2) [EP] | 960, 4339 | |
| 3 | MS (3) [EP] | 960, 4339 | |
| 4 | ML (1-1) [EP] | 980 3378 | ○ |
| 5 | ML (1-2) [EP] | 980 3378 | ○ |
| 6 | ML (1-3) [EP] | 980 3378 | × |
| 7 | ML (1-4) [EP] | 980 3378 | × |
| 8 | ML (2-1) [EP] | 980 3378 | ○ |
| 9 | ML (2-2) [EP] | 980 3378 | × |
| 10 | ML (2-3) [EP] | 980 3378 | × |
| 11 | ML (2-4) [EP] | 980 3378 | × |
| 12 | MS (1) [EP] | 960, 4339 | ○ |
| 13 | MS (2) [EP] | 960, 4339 | ○ |
White colonies are not inserted R0011 but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of lacI gene.
Error PCR (Retry)
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template SSam7,ΔTMD1-E0840 failed (50ng/µL) | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| SSam7,ΔTMD1-E0840 (2) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 25 cycles |
| 68℃ | 4min | |
| Add DpnI 2µL | ||
| Incubate | 1h | |
| 4℃ | forever | |
Transformation
| Name | Well | Sample | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | - | 4 µL | 50 | 54 | LB (Kan+) | 08/06-08/09 | ○ |
| SSam7,ΔTMD1-E0840 (2) | - | 4 | 50 | 54 | ○ | ||
| I20260 | 2-17-F | 2 | 50 | 52 | ○ | ||
| 2-I-5 | 2 | 50 | 52 | LB (Amp+) | ○ |
Monday, August 9 By: Wataru, Tomonori, Ken, Takuya
Miniprep
| Name | concentration |
|---|---|
| MS | 116.2 ng/µL |
| ML | 146.6 |
Transfotrmation
| Sample | Sample | Competent Cell | Total | Plate | Incuvation | Results | |
|---|---|---|---|---|---|---|---|
| MS | 2 µL | KRX | 50 | 52 | LB (Kan+) | 08/09 18:00-08/10 12:00 | ○ |
| ML | 2 | KRX | 50 | 52 | ○ | ||
Restriction Eigestion and Ethanol Precipitation
To use R0011 for next ligation, we digested it by EcoRI and PstI
| Name | Sample | 10x Buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| R0011 [EP] | 50 | 6 | 0.6 | EcoRI | 0.5 | PstI | 0.5 | 2.4 | 60 | At 37℃ 08/09 16:20-18:20 |
After restriction enzyme digestion, we did ethanol precipitation.
Ligation and Transformation
| Name | Sample | Competent cell | Total | Plate | Incuvation | Colony | |
|---|---|---|---|---|---|---|---|
| R0011 [pSB4K5, KRX] | 2 µL | KRX | 50 | 52 | LB (Kan+) | 08/09 20:00-08/10 09:00 | ○ |
| R0011 [pSB4K5</partrinfo>, C2] | 2 | C2 | 50 | 52 | ○ | ||
Tuesday, August 10 By: Wataru, Tomonori, Ken, Fumitaka
Culture
Cultured I20260 [pSB4K5, ML, R0011 [pSB4K5, KRX], and R0011 [pSB4K5</partrinfo>, C2].
Minprep
| Name | Concentration |
|---|---|
| SSam7,ΔTMD1-E0840 (1-1) | 9.9 ng/µL |
| SSam7,ΔTMD1-E0840 (1-2) | 27.3 |
| SSam7,ΔTMD1-E0840 (2-1) | 43.2 |
| SSam7,ΔTMD1-E0840 (2-2) | 34.7 |
Culture and Master Plate
At 37℃ 08/09 18:00-08/10 9:00
Wednesday, August 11 By: Wataru, Naoi, Ken, Takuya
| No. | Medium | Cloud | Incubation |
|---|---|---|---|
| 1 | Kanamycin | ○ | At 37℃, 08/10 20:00-08/11 9:00 |
| Ampicillin | × | ||
| 2 | Kanamycin | ○ | |
| Ampicillin | ○ | ||
| 3 | Kanamycin | ○ | |
| Ampicillin | × | ||
| 4 | Kanamycin | ○ | |
| Ampicillin | × | ||
| 5 | Kanamycin | ○ | |
| Ampicillin | × | ||
| 6 | Kanamycin | ○ | |
| Ampicillin | ○ | ||
| 7 | Kanamycin | ○ | |
| Ampicillin | × |
Discussion: About sample 1, 3, 4, 5 and 7, lac promoter was correctly inserted in low copy plasmid. About sample 2 and 6, low copy plasmid and vector derived from lac promoter were ligated. We decided to use sample 1 or 3.
Miniprep of R0011 [pSB4K5, C2], SRRz 1', 3'
| Name | Concentration |
|---|---|
| R0011 [pSB4K5, C2] (1) | 31.2 ng/µL |
| R0011 [pSB4K5, C2] (3) | 29.9 |
Restriction Digestion and electrophoresis of R0011 [pSB4K5, C2]
| Name | EcoRI | PstI |
|---|---|---|
| 1 | 0.2 | - |
| 2 | - | 0.2 |
| 3 | 0.2 | 0.2 |
| N | - | - |
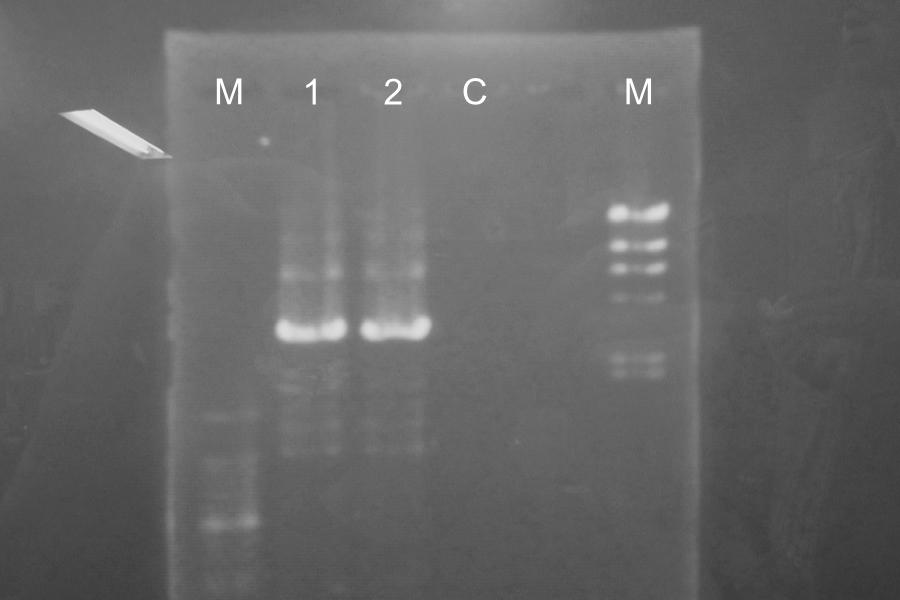
| No. | Name | Length | Results |
|---|---|---|---|
| 1 | R0011 [pSB4K5, C2] (1-1) | ||
| 2 | R0011 [pSB4K5, C2] (1-2) | ||
| 3 | R0011 [pSB4K5, C2] (1-3) | ||
| 4 | R0011 [pSB4K5, C2] (1-N) | ||
| 5 | R0011 [pSB4K5, C2] (2-1) | ||
| 6 | R0011 [pSB4K5, C2] (2-2) | ||
| 7 | R0011 [pSB4K5, C2] (2-3) | ||
| 8 | R0011 [pSB4K5, C2] (2-N) |
Each enzyme correctly cut samples.
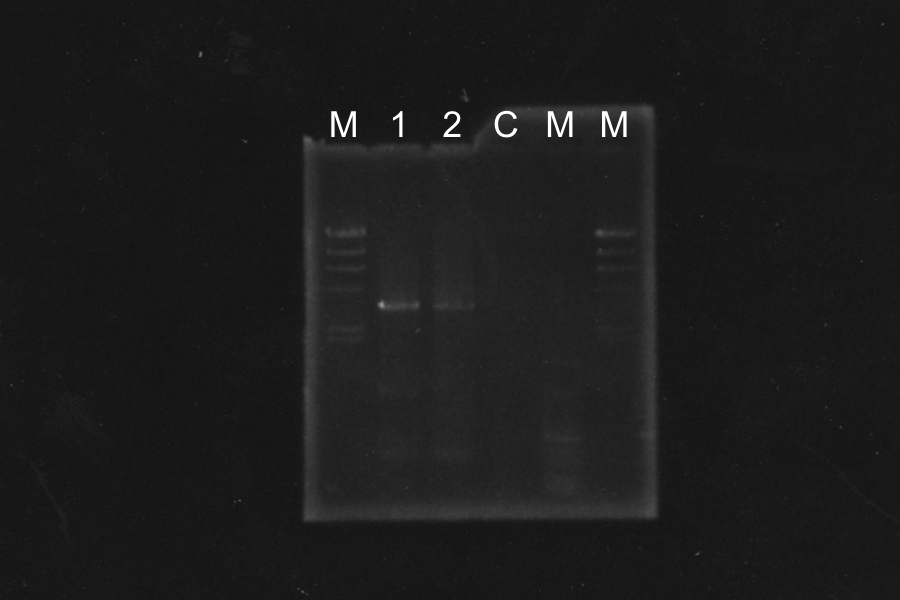
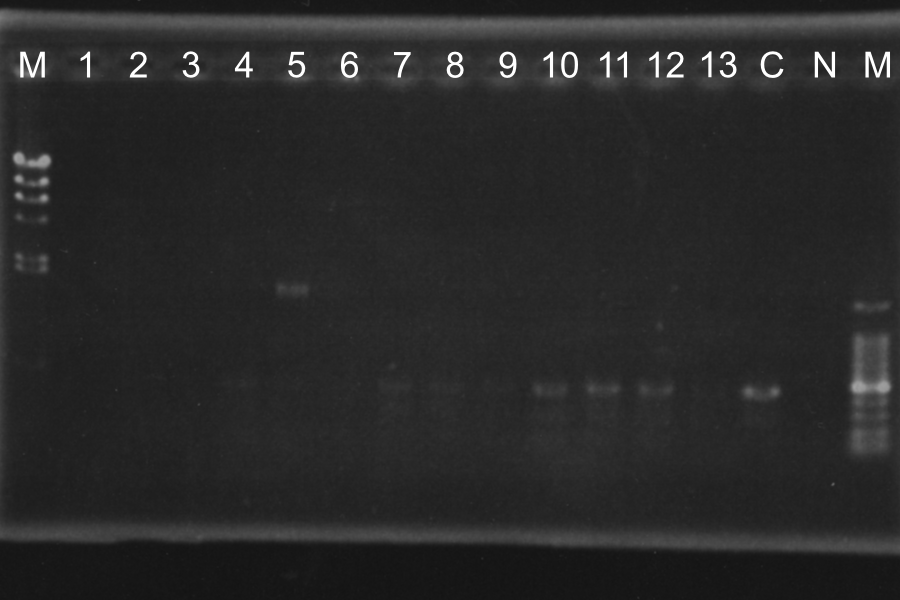
Screening PCR of SRRz
| No. | Name | Results |
|---|---|---|
| 1 | None | |
| 2 | Control B0015 | |
| 3 | Control J06702 | |
| 4 | Control B0015 | |
| 5-24 | SRRz-B0015 | × |
Marker: Lambda Marker
Discussion: All of the sample were self-ligation of DT. SRRz weren't inserted.
Thursday, August 12 By: Wataru, Ken
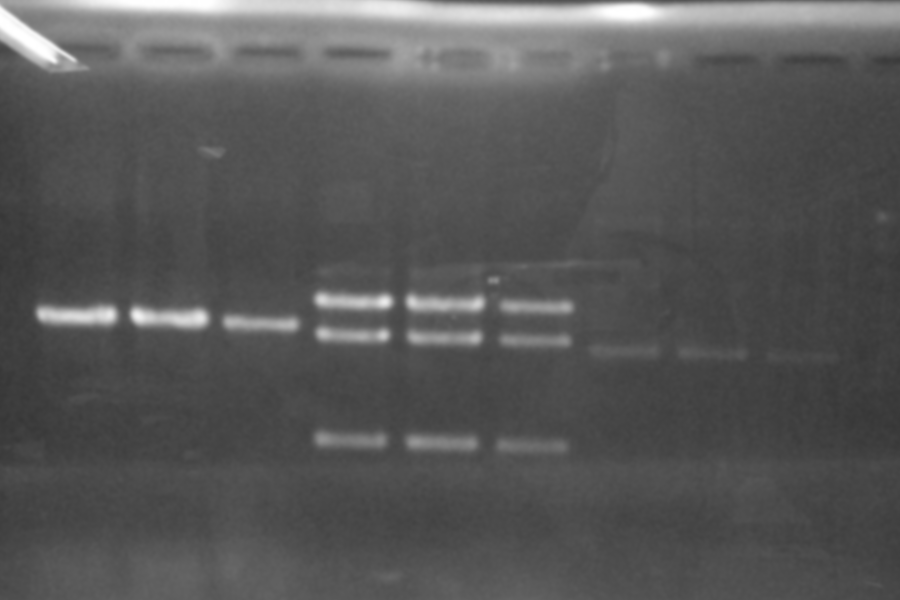
Restriction Digestion and electrophoresis of B0015
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | XbaI 1 | XbaI 2 | SpeI | PstI 1 | PstI 2 | Water | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 1 | 0.1 | 0.2 | - | - | - | - | - | 5.7 | 10 |
| 2 | 3 | 1 | 0.1 | - | 0.2 | - | - | - | - | 5.7 | 10 |
| 3 | 3 | 1 | 0.1 | - | - | 0.2 | - | - | - | 5.7 | 10 |
| 4 | 3 | 1 | 0.1 | - | - | - | 0.2 | - | - | 5.7 | 10 |
| 5 | 3 | 1 | 0.1 | - | - | - | - | 0.2 | - | 5.7 | 10 |
| 6 | 3 | 1 | 0.1 | - | - | - | - | - | 0.2 | 5.7 | 10 |
| N | 3 | 1 | 0.1 | - | - | - | - | - | - | 5.9 | 10 |
Maker: Lambda, 100bp
Discussion: Each enzyme correctly cut each sample and was active.
Thursday, August 19 By: Wataru, Tomo, Ken
Miniprep of SSam7,ΔTMD1-E0840
| Name | Concentration |
|---|---|
| SSam7,ΔTMD1-E0840 | 29.6 ng/µL |
Point mutation PCR of SSam7,ΔTMD1-E0840
| Name | Template | 10xbuffer | dNTPs | MgSO4 | Primer Fwd. | Primer Rev. | MilliQ | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| SΔTMD1-E0840 (1) | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 31.5 | 1 | 50 |
| SΔTMD1-E0840 (2) | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 31.5 | 1 | 50 |
| Control | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 32.5 | - | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30cycles |
| 55℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | forever |
Restriction Digestion by DpnI from 17:50 to 18:50
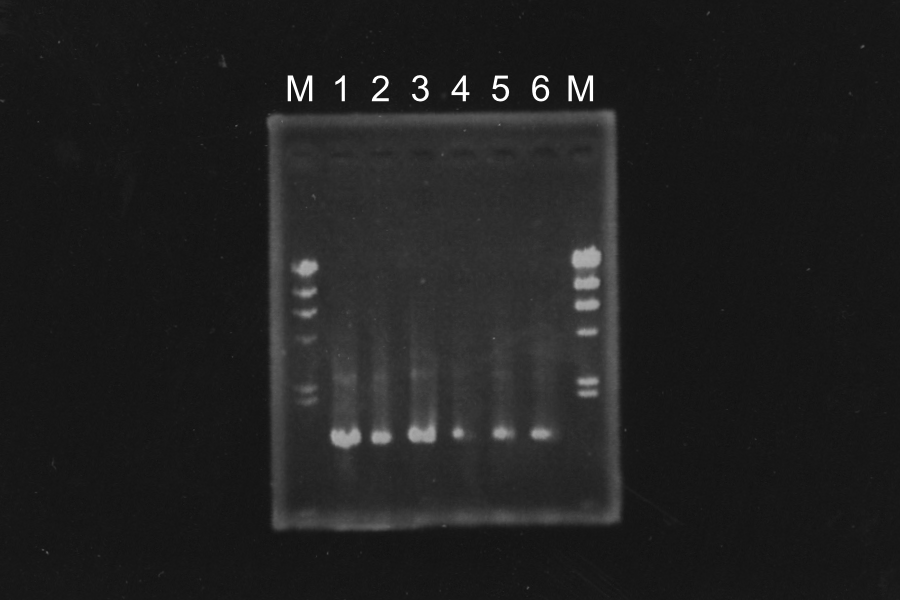
Electrophoresis
| Name |
|---|
| SΔTMD1-E0840 (1) |
| SΔTMD1-E0840 (2) |
| Control |
Marker: Lambda, 100bp
Ligation and Transformation
| Name | Colony |
|---|---|
| SΔTMD1-E0840 (1) | ○ |
| SΔTMD1-E0840 (2) | ○ |
| Control | × |
Friday, August 20 By: Wataru, Ken
Making Culture and Master Plate of SΔTMD1-E0840
Miniprep
| Name | Concentration |
|---|---|
| B0015 | 41.1 ng/µL |
PCR of SRRz
| Name | 10xBuffer | MgS04 | dNTP | Template | Primer Fwd. | Primer Rev. | MilliQ | KOD plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| SRRzSam7 (1) | 5 µL | 3 | 5 | 5 | F1 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (2) | 5 | 3 | 5 | 5 | F2 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (3) | 5 | 3 | 5 | 5 | F1 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (4) | 5 | 3 | 5 | 5 | F2 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (5) | 5 | 3 | 5 | 5 | F1 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (6) | 5 | 3 | 5 | 5 | F2 | 1.5 | 1.5 | 28 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30cycles |
| 55℃ | 30s | |
| 68℃ | 2min | |
| 4℃ | forever |
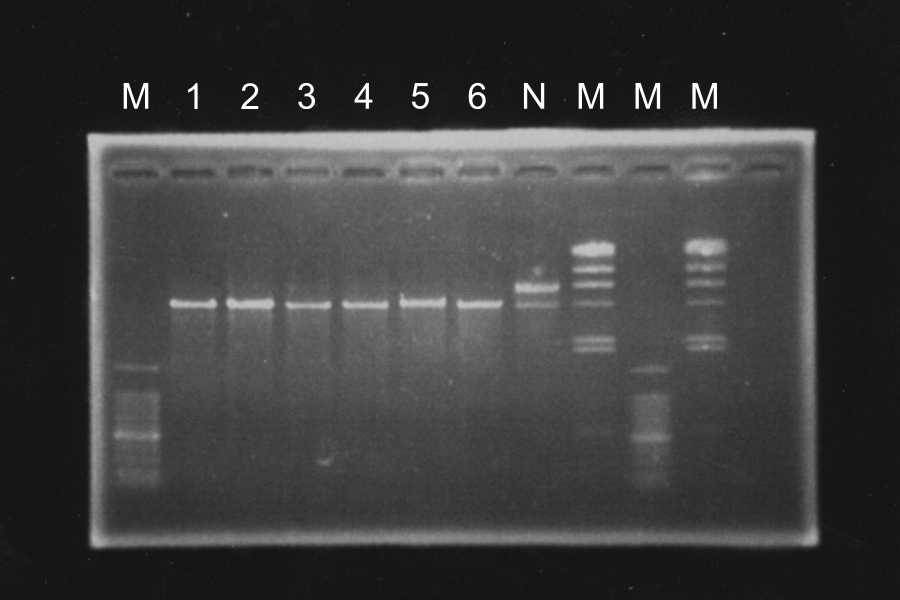
Electrophoresis
| Name |
|---|
| SRRzSam7 (1) |
| SRRzSam7 (3) |
| SRRz Sam7(5) |
| SRRzSam7 (2) |
| SRRzSam7 (4) |
| SRRzSam7 (6) |
Discussion: Primer F1 might be better than F2, because the bands of 1, 2 and 3 were clearer. We decided to use sample 1 and 3. Their bands were clearer in the three.
PCR Purification
| Name | Concentration |
|---|---|
| SRRzSam7 (1) | 134.0 ng/µL |
| SRRzSam7 (3) | 69.0 |
Restriction Digestion
| Name | Sample | 10xBuffer | 100xBuffer | EcoRI | XbaI | SpeI | MilliQ | Total | Incubation |
|---|---|---|---|---|---|---|---|---|---|
| B0015 [EX] | 50 µL | 6 | 0.6 | 0.4 | 0.4 | - | 2.6 | 60 | 17:45-18:45 |
| SRRzSam7 (1) [EP] | 50 | 6 | 0.6 | 0.4 | - | 0.4 | 2.6 | 60 | |
| SRRzSam7 (3) [EP] | 50 | 6 | 0.6 | 0.4 | - | 0.4 | 2.6 | 60 |
Purification
| Name | Concentration |
|---|---|
| SRRzSam7 (1) [EP] | 109.0 ng/µL |
| SRRzSam7 (2) [EP] | 110.0 |
| B0015 | 25.5 |
Ligation and Transformation
Monday, August 23 By: Wataru, Tomo, Ken, Fumitaka, Tasuku
Miniprep
| Sample number | Concentration |
| SΔTMD1-E0840 (1-1) | 58.9 ng/µL |
| SΔTMD1-E0840 (2-2) | 49.9 |
Sequence
Sample: SΔTMD1-E0840 (1-1). SΔTMD1-E0840 (2-2), MS Discussion: The sequencing was in success and the results were desirable. It meant the point mutation was succeeded and sequence of MS was confirmed. We decided to use SΔTMD1-E0840.
Screening PCR of SRRzSam7-B0015
| 90℃ | 10min | |
| 94℃ | 30s | 35cycles |
| 50℃ | 30s | |
| 72℃ | 1.5min | |
| 72℃ | 4min | |
| 4℃ | hold |
| No. | Name |
|---|---|
| 1-13 | SRRzSam7-B0015 |
| C | Control: B0015 |
| N | None |
Marker: Lambda, 100bp
Discussion: We found the band; about 200bp, and it meant the lligation was completed successfully.
Deletion PCR of SΔTMD1-E0840 (2-2)
| Name | Sample | 10xBuffer | dNTPs | Primer Fwd. | Primer Rev. | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1) | 2 µL | 5 | 5 | 1.5 | 1.5 | 1 | 35 | 1 | 50 |
| rrSΔTMD1-E0840 (2) | 2 | 5 | 5 | 1.5 | 1.5 | 1 | 35 | 1 | 50 |
| rrSΔTMD1-E0840 (Control) | 2 | 5 | 5 | 1.5 | 1.5 | 1 | 35 | - | 50 |
| 94℃ | 2min | |
| 94℃ | 10s | 35cycles |
| 56℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | forever |
Restriction Digestion (DpnI)
| Sample | DpnI | Total | Incubation |
|---|---|---|---|
| 25 µL | 1 | 26 | 19:00-20:10 |
Ligation
| Name | Sample | Water | Ligation high | T4 Kinase | Total | Incubation |
|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1) | 3 µL | 6 | 5 | 1 | 15 | 20:15-21:15 |
| rrSΔTMD1-E0840 (2) | 3 | 6 | 5 | 1 | 15 | |
| rrSΔTMD1-E0840 (Control) | 3 | 6 | 5 | 1 | 15 |
Transformation
Tuesday, August 24 By: Ken, Tomo, Tasuku, Takuya
Retry of deletion PCR of SΔTMD1-E0840
| Name | Sample | 10xBuffer | dNTPs | MgSO4 | Primer1 | Primer2 | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1) | 2 µL | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| rrSΔTMD1-E0840 (2) | 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| Control | 2 | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| 94℃ | 2min | |
| 94℃ | 10s | 35cycles |
| 58℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | hold |
Restriction Digestion (DpnI)
14:15-15:15
Electrophoreis
| Lane | Name |
|---|---|
| 1 | rrSΔTMD1-E0840 (1) |
| 2 | rrSΔTMD1-E0840 (3) |
| C | rrSΔTMD1-E0840 (Control) |
Marker: 100bp, Lambda.
We found the band of sample 1 and 2 about 3000bp and there wasn't the band of sample control. So, we confirmed the PCR and Restriction Digestion were completed successfully.
Ligation
Point mutation of SRRz
| Name | 10x | dNTPs | MgSO4 | Primer1 | Primer2 | Template | Water | KOD-plus- | total |
|---|---|---|---|---|---|---|---|---|---|
| SRRzSam7-B0015 (1) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| SRRzSam7-B0015 (2) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| SRRzSam7-B0015 (Control) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | hold |
Restriction Digestion (DpnI), Electrophoresis and Ligation
We could find point mutation PCR and restriction enzyme of DpnI was done.
PCR of E0240
| Sample | 10xBuffer | dNTPs | MgSO4 | VF2 | VR | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| E0240 (1) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31.5 | 1 | 50 |
| E0240 (2) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31.5 | 1 | 50 |
PCR Purification
| Name | Concentration |
|---|---|
| E0240 (1) | 5.5 x 50 ng/µL |
| E0240 (2) | 5.2 x 50 |
Restriction Digestion (EcoRI, PstI) and Gel Extraction
| Name | Concentration |
|---|---|
| E0240 (1) | 28.8 ng/µL |
| E0240 (2) | 26.4 |
Transformation
Wednesday, August 25 By:Ken, Tomo, Kazuya, Tasuku, Takuya
Making culture and Master plate
| Name | Colony |
|---|---|
| rrSΔTMD1-E0840 (1) | ○ |
| rrSΔTMD1-E0840 (2) | ○ |
| rrSΔTMD1-E0840 (Control) | × |
| SRRzSam7-B0015 (1) | ○ |
| SRRzSam7-B0015 (2) | ○ |
| SRRzSam7-B0015 (Control) | × |
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 29.0 ng/µL |
Restriction Digestion
| Sample name | Template | 10xbuffer | 100xbuffer | EcoRI | SpeI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|---|
| pSB4K5 | 50 | 6 | 0.6 | 0.4 | 0.4 | - | 2.6 | 60 |
| R0011 [pSB4K5] | 10 | 4 | 0.4 | - | 0.3 | 0.3 | 25 | 40 |
Purification
| Sample Name | Concentration |
| pSB4K5 | 18.4 ng/µL |
| R0011 [pSB4K5] | 8.6 |
Ligation of E0240 and pSB4K5, Transformation
Thursday, August 26 By:Ken, Tomo, Kazuya, Tasuku, Takuya, Fumitaka
Miniprep
| Sample name | Concentration |
| J23116 (RPU0.7) | 44.5 ng/µL |
Restriction Digestion
| Name | Template | 10xbuffer | 100xbuffer | SpeI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|
| J23116 (RPU0.7) | 25 | 4 | 0.4 | 0.3 | 0.3 | 10 | 40 |
Purification of
| Name | Concentration |
|---|---|
| J23116 (RPU0.7) | 49.8 ng/µL |
Friday, August 27 By: Ken, Tomo, Kazuya, Fumitaka
Making master plate of E0240 [pSB4K5]
| Sample Name | Concentration |
| rrSΔTMD1-E0840 (1-2) | 20.9 ng/µL |
| SRRz-B0015 (1-1) | 16.4 |
Restriction Digestion
| Name | Template | 10xbuffer | 100xbuffer | XbaI | PstI | Water | Total | Incubation |
|---|---|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1-2) | 45 µL | 6 | 0.6 | 0.3 | 0.3 | 7.8 | 60 | 13:20-14:20 |
| SRRz-B0015 (1-1) | 45 | 6 | 0.6 | 0.3 | 0.3 | 7.8 | 60 |
Purification
| rrSΔTMD1-E0840 (1-2) | 44.7 ng/µL |
| SRRz-B0015 (1-1) | 56.1 |
Ligation and Transformation
| Name |
|---|
| R0011-rrSΔTMD1-E0840 (1-2) |
| J23116 (RPU0.7)- rrSΔTMD1-E0840 (1-2) |
| R0011-SRRz-B0015 (1-1) [pSB4K5] |
Monday, August 30 By: Tomonori, Kazuya, Tasuku, Ken
Making culture and Master plate
| Name | Colony |
|---|---|
| R0011-rrSΔTMD1-E0840 | Many colonies |
| R0011-rrSΔTMD1-E0840 (Control) | Some colonies |
| J23116 (RPU0.7)- rrSΔTMD1-E0840 | Many colonies |
| J23116 (RPU0.7)- rrSΔTMD1-E0840 (Control) | Many colonies |
| R0011-SRRz-B0015 (1-1) [pSB4K5] | No colony |
| R0011-SRRz-B0015 (1-1) [pSB4K5] (Control) | No colony |
Discussion: There ware some colonies, which emitted green light, on the plate 1. So, we cultured those colonies on master plate. On the plate 5 and 6, even though we used KRX, which is able to repress lac promoter, colonies might be dead. However, we still have to do some experience so that we confirm lac promoter cannot repress enough and E. coli cannot survive.
Tuesday, August 31 By: Tomonori, Takuya Y., Kazuya, Tasuku, Takuya, Ken
Miniprep
| Name | Concentration |
|---|---|
| J23105 (RPU0.3) | 48.5 ng/µL |
| R0011-rrSΔTMD1-E0840 | 107.3 |
Restriction Digestion
Gel Extraction of R0011-rrSΔTMD1-E0840 (Electrophoresis for 45min)
Discussion: There were two band at the bottom of the gel. It was too long -45min-, and insert and vector might be contaminated. But we went on next operation.
Purification of J23105 (RPU0.3) and R0011-rrSΔTMD1-E0840
| Name | Concentration |
|---|---|
| J23105 (RPU0.3) | 5.8 ng/µL |
| R0011-rrSΔTMD1-E0840 | 7.8 |
Ligation and Transformation
| Insert | Vector |
| R0011-rrSΔTMD1-E0840 | J23105 (RPU0.3) |
Wednesday, September 1 By: Tomonori, Kazuya, Tasuku, Fumitaka, Ken
Making culture and Master plate
| Name | Colony |
|---|---|
| R0011-rrSΔTMD1-E0840-J23105 (RPU0.3) | Many colonies |
| R0011-rrSΔTMD1-E0840-J23105 (RPU0.3) (Control) | Many colonies |
Screenig PCR of R0011-rrSΔTMD1-E0840-J23105 (RPU0.3)
- Sample: 1-13
- Control: Positive (B0015)
- Maker: lambda, 100
Discussion: All of the sample except sample 10 might be self-ligation products of J23105 (RPU0.3).
Miniprep
| SRRz-B0015 (1-1) | 33.8 ng/µL |
| pSB4K5 | 56.0 |
Restriction Digestion of SRRz and pSB4K5
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | PstI | Water | Total | Incubation |
|---|---|---|---|---|---|---|---|---|
| SRRz-B0015 (1-1) | 20 µL | 4 | 0.4 | 0.3 | 0.3 | 15 | 40 | 13:25-14:30 |
| pSB4K5 | 20 | 4 | 0.4 | 0.3 | 0.3 | 15 | 40 |
Purification
| SRRz-B0015 (1-1) | 6.5 ng/µL |
| pSB4K5 | 16.8 |
Ligation and transformation
- Insert: SRRz-B0015 (1-1)
- Vector: pSB4K5
Thursday, September 2 By: Tomonori, Tomo, Takuya, Ken
Making culture and Master plate
| SRRz-B0015 (1-1) [pSB4K5] | 13 colonies |
| SRRz-B0015 (1-1) [pSB4K5] (Control) | 13 colonies |
Screening PCR of rSRRz low
Sample: (1-13) SRRz-B0015 (1-1) [pSB4K5] Maker: Lambda, 100 Control: Positive (B0015), Neganive Discussion: From sample 1, two vectors might be ligated. Sample 3 and 4, SRRz-B0015 might be inserted in low copy plasmid correctly. Sample 11, it might be the self-ligation product of low copy plasmid. Anyway, we decided to culture those 4 colonies on master plate.
Friday, September 3 By: Tomonori, Tomo, Kazuya, Tasuku, Fumitaka, Ken
Making culture
- R0011-rrSΔTMD1-E0840 (1)
- R0011-rrSΔTMD1-E0840 (3)
- rrSΔTMD1-E0840 (1-1)
- rrSΔTMD1-E0840 (1-2)
- SRRz-B0015 (1-1) [pSB4K5]
- SRRz-B0015 (1-2) [pSB4K5]
- ML
Monday, September 6 By: Wataru, Tomo, Kazuya, Ken
Sequence Analysis
- R0011-rrSΔTMD1-E0840 (A)
- R0011-rrSΔTMD1-E0840 (B)
- SRRz-B0015 [pSB4K5] (A)
- SRRz-B0015 [pSB4K5] (B)
- rrSΔTMD1-E0840 (1-1)
- rrSΔTMD1-E0840 (1-2)
R0011-rrSΔTMD1-E0840 (A), SRRz-B0015 [pSB4K5] (A) is correct.
Miniprep
| Name | Concentration |
|---|---|
| SRRz | 29.6 ng/µL |
| R0011-rrSΔTMD1-E0840 | 70.2 |
| J23105 (RPU0.3) | 75.3 |
| rrSΔTMD1-E0840 (1-1) | 30.3 |
Restriction Digestion
- J23105 (RPU0.3): SpeI, PstI
- rrSΔTMD1-E0840: XbaI, PstI
Gel Extraction
| Name | Concentration |
|---|---|
| J23105 (RPU0.3) [SP] | 20ng / 10µL |
| rrSΔTMD1-E0840 | 100ng / 1µL |
Ligation
| Vector | Insert | Ligation High | Total | Incubation | ||
|---|---|---|---|---|---|---|
| J23105 (RPU0.3) [SP] | 1 µL | rrSΔTMD1-E0840 [XP] | 1 | 2 | 4 | 30min |
Transformation
| Name | Competent Cell | Product of Ligation | |
|---|---|---|---|
| J23105 (RPU0.3)-rrSΔTMD1-E0840 | 50 µL | 4 |
Tuesday, September 7 By: Wataru, Ken
Insert Check
We did colony PCR, and three colonies were inserted rrSΔTMD1-E0840. So we succeeded in making J23105 (RPU0.3)-rrSΔTMD1-E0840.
Thirsday, September 9 By: Wataru, Ken
Culture
Culture pSB4K5 and SRRzSam7
Friday, September 10 By: Wataru, Ken
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 48.8 |
| SRRzSam7 | 33.4 |
Mutagenesis
We lost SRRz-B0015, so we decided to retry point mutation.
| Name | MgS04 | dNTP | 10xBuffer | Template | KOD | MilliQ | Total |
|---|---|---|---|---|---|---|---|
| SRRz (1) | 3 | 5 | 5 | 1.5 | 1 | 34 | 50 |
| SRRz (2) | 3 | 5 | 5 | 1.5 | 1 | 34 | 50 |
| Control | 3 | 5 | 5 | 1.5 | 1 | 34 | 50 |
| 94℃ | 1min | |
| 94℃ | 5s | 25 cycles |
| 55℃ | 30s | |
| 68℃ | 3min 40s | |
| 4℃ | Forever |
After digestion by DpnI, Ligation and Transformation.
Sunday, September 12 By: Wataru
Culture
Culture SRRz (1), (2), (3) from original plate.
Monday, September 13 By: Wataru, Ken
Miniprep
| Name | Concentration |
|---|---|
| SRRz (1) | 61.3 ng/µL |
| SRRz (2) | 59.3 |
| SRRz (3) | 69.3 |
Sequence Analysis
- SRRz (1), (2), (3)
SRRz (1), (2), (3) are correct.
Restriction Digestion
- J23105 (RPU0.3)-rrSΔTMD1-E0840: EcoRI, SpeI
- R0011: EcoRI, XbaI
Ligation
J23105 (RPU0.3)-rrSΔTMD1-E0840 [ES] + R0011 [EX]
Transformation
Tuesday, September 14 By: Ken, Wataru
Colony PCR
We did Colony PCR, and we succeeded in making J23105 (RPU0.3)-rrSΔTMD1-E0840-R0011
Sequence Analysis
From the product of Colony PCR.
- J23105 (RPU0.3)-rrSΔTMD1-E0840-R0011
J23105 (RPU0.3)-rrSΔTMD1-E0840-R0011 is correct.
Tuesday, September 30 By: Ken
Miniprep
| Name | Concentration |
|---|---|
| LTC (1) | 84.6 ng/µL |
| LTC (2) | 97.6 |
| LTC (3) | 127.4 |
| LTC (4) | 85.4 |
| LTC (5) | 70.0 |
Friday, October 1 By: Ken
Sequence Analysis
Analyzed LTC 1-5. The sequencing on each sample was in success. The results of sequencing with VF2 were desirable, but, on the other, only one of those with VR was desirable, the others were found bad sequencing. We decided to use LTC 2.
Saturday, October 2 By: Ken
Miniprep
| Name | Concentration |
|---|---|
| SRRz-low | 162.3 ng/µL |
| CTL | 60.8 ng/µL |
Restriction Digestion
- LTC (EcoRI-SpeI)
- CTL (EcoRI-SpeI)
- SRRz low (EcoRI-XbaI)
Sunday, October 3 By: Ken
Miniprep
| Name | Concentration |
|---|---|
| SRRz-low | 237.3 ng/µL |
| CTL | 138.8 |
| LTC | 441.2 |
| CT | 252.1 |
Restriction Digestion
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | XbaI | SpeI | CIAP | Water | Total |
|---|---|---|---|---|---|---|---|---|---|
| SRRz-low | 7 | 4 | 0.4 | 0.3 | 0.3 | - | 1 | 27 | 40 |
| CTL | 10 | 4 | 0.4 | 0.3 | - | 0.3 | - | 25 | 40 |
| LTC | 4 | 4 | 0.4 | 0.3 | - | 0.3 | - | 31 | 40 |
Concentration(after purification)
| Name | Concentration |
|---|---|
| SRRz-low(E-X) | 36.9 ng/µL |
| CTL(E-S) | 25.0 |
| LTC(E-S) | 64.3 |
Ligation and Transformation
Ligation and transformation of CTL-SRRz(CTLS) and LTC-SRRz(LTCS).
| Name | Colony |
|---|---|
| CTLS | Some colonies |
| LTCS | Some colonies |
Monday, October 4 By: Ken
Making culture
Cultured CTLS and LTCS with LB-Kan(+/- IPTG).
Tuesday, October 5 By: Ken
Results of Screening
The propagation wasn't observed on some samples on CTLS cultured with M9-Kan +IPTG. It meant that the construction of CTLS was in success. However, the growth was observed on all of samples of LTCS. So, we decided to retry the construction of LTCS.
Miniprep
| Name | Concentration |
|---|---|
| LT | 387.1 ng/µL |
Making culture
Cultured CTLS.
Wednesday, October 6 By: Ken
Thursday, October 7 By: Ken
Miniprep
| Name | Concentration |
|---|---|
| SΔTMD1 | 84.1 ng/µL |
| lac-SRRz | 172.5 |
Restriction Digestion
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|
| LT | 4 | 4 | 0.4 | 0.4 | 0.4 | 30.8 | 40 |
| CT | 6 | 4 | 0.4 | 0.4 | 0.4 | 28.8 | 40 |
| CTL | 11 | 4 | 0.4 | 0.4 | 0.4 | 23.8 | 40 |
| LTC | 4 | 4 | 0.4 | 0.4 | 0.4 | 30.8 | 40 |
| SRRz | 7 | 4 | 0.4 | 0.4 | 0.4 | 27.8 | 40 |
| SΔTMD1 | 17 | 4 | 0.4 | 0.4 | 0.4 | 17.8 | 40 |
Concentration after purification
| Name | Concentration |
|---|---|
| LT | 107.9 ng/µL |
| CT | 56.9 |
| CTL | 37.1 |
| LTC | 101.9 |
| SRRz | 55.7 |
| SΔTMD1 | 102.9 |
Friday, October 8 By: Ken
Screening
Screened lac-SRRz 1-7(LB-Tc with IPTG 1.5mM). Except for sample 4, the propagation could be observed. So, we decided to use sample 4.
Restriction Digestion of pSB1C3
| Template | 10xbuffer | 100xbuffer | EcoRI | PstI | Water | Total |
|---|---|---|---|---|---|---|
| 5 | 4 | 0.4 | 0.4 | 0.4 | 28.4 |
Concentration after purification
| Name | Concentration |
|---|---|
| pSB1C3(E-P) | 16.1 ng/µL |
Ligation and Transformation
Inserted LTC, CTL, T, SRRz, LT or CT to pSB1C3.
| Name | Colony |
|---|---|
| LTC | Some colonies |
| CTL | No colony |
| T | Some colonies |
| SRRz | Some colonies |
| LT | Some colonies |
| CT | Some colonies |
Saturday, October 9 By: Ken
Making culture
Cultured LTC, T, SRRz, LT, and CT with LB (Cp/Amp+).
Restriction digestion of LTC and SRRz-low
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | SpeI | Water | Total |
|---|---|---|---|---|---|---|---|
| LTC | 3.5 | 4 | 0.4 | 0.6 | 0.6 | 30.9 | 40 |
| SRRz-low | 4 | 4 | 0.4 | 0.6 | 0.6 | 30.4 | 40 |
Ligation and Transformation of LTCS
| Name | Colony |
|---|---|
| LTCS | Many colonies |
| control | few colonies |
Used Top 10.
Sunday, October 10 By: Ken
Screening
Screened LTC, T, SRRz, LT, and CT. Except for LT1, the propagation could not be observed on each sample with LB (Amp+).
Miniprep
| Name | Concentration |
|---|---|
| LTC1 | 210.6 ng/µL |
| T2 | 193.2 |
| SRRz3 | 208.9 |
| LT2 | 195.7 |
| CT1 | 219.9 |
| CTLS4-2 | 231.0 |
| LTCS1 | 50.6 |
Restriction Digestion of LTC, T, SRRz, LT, CT, and CTLS
| Template | 10xBuffer | 100xBuffer | EcoRI | PstI | MilliQ | Incubation |
|---|---|---|---|---|---|---|
| 2 µL | 1 | 0.1 | 0.2 | 0.2 | 6.5 | 60min |
Electrophoreis
- Lane 1, 8: Lambda, 100bp Marker
- Lane 2-7: LTC, T, SRRz, LT, CT, CTLS
We could observe that the each part was correctly inserted to the pSB1C3.
Retry - Transformation of CTL (pSB1C3) and LTCS
Used KRX.
Deletion PCR of T2
| Name | Template | 2xBuffer | dNTPs | MgSO4 | Primer1 | Primer2 | Primer3 | KOD-FX | MilliQ | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| GFP-Deletion1, 2 | 0.25 µL | 25 | 10 | 3 | 1.5 | - | 1.5 | 1 | 8.75 | 50 |
| GFP-DT-Deletion1, 2 | 0.25 µL | 25 | 10 | 3 | - | 1.5 | 1.5 | 1 | 8.75 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 59℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | Hold |
Primer:
- ccaggcatcaaataaaacgaaaggctc
- tactagtagcggccgctgc
- ctctagtattattgatttctaccatcttctactcc
Monday, October 11 By: Ken
Restriction Digestion, Electrophoresis, Ligation and Transformation of PCR products
| Template | DpnI | Incubation |
|---|---|---|
| 25 µL | 1 | 60min |
| Lane | Name |
|---|---|
| 1,6 | Lambda, 100bp Marker |
| 2 | GFP-Deletion1 |
| 3 | GFP-Deletion2 |
| 4 | GFP-DT-Deletion1 |
| 5 | GFP-DT-Deletion2 |
Deletion PCR was well performed.
| Template | Ligation High | T4 Kinase | Incubation |
|---|---|---|---|
| 9 µL | 5 | 1 | 90min |
| Name | Colony |
|---|---|
| SΔTMD1-dT | Many colonies |
| SΔTMD1 | Many colonies |
- Competent Cell: TOP10
Sequence
Sequenced LTC1, T2, SRRz3, LT2, CT1, CTLS4-2 and LTCS1.
| Template | 5xBuffer | Primer | Big Dye | MilliQ | Total |
|---|---|---|---|---|---|
| (200ng) | 2 µL | 1 | 0.5 | 6.5 | 10 |
Primer:
- VF2
- VR
- aggtgatgcaacatacggaaaacttacc
- tgctgggattacacatggcatggatg
- ttcctcgatatgctggcgtggtc
Used Primer 1 or 2 to each sample, and Primer 3, 4, or 5 only to CTLS4-2 and LTCS1.
| 96℃ | 1min | |
| 96℃ | 10s | 40 cycles |
| 50℃ | 5s | |
| 60℃ | 2min 5s | |
| 4℃ | Hold |
Except for CTLS4-2 and LTCS1 with Primer2, the sequencing of each sample were well performed. And LCT1, T2, LT2, and CT1 were confirmed that the sequence were correct. There was, however, deletion of some base pairs on lactose promoter [R0011]. of CTLS402.
- Result: AATTGTGAGCGGATAACAAGATACTGAGCACA
- BBa_R0011: AATTGTGAGCGGATAACAATTGACATTGTGAGCGGATAACAAGATACTGAGCACA
There is repeated sequence on lactose promoter and the one was deleted. So, we supposed that the homologous recombination was occureed. From this result, we could say that the function check of CTLS was failed because SRRz gene wasn't induced by IPTG.
Making culture of LTCS and CTL
Tuesday, October 12 By: Ken
Screening of CTL
The propagation could not be observed with LB-Amp and could be observed with LB-Cp. It meant that the part, CTL, was correctly inserted to the plasmid, pSB1C3.
Making culture
Cultured two samples on each part: SΔTMD1-dT and SΔTMD1. Named T1,3,4 and 6.
Wednesday, October 13 By: Ken
Thursday, October 14 By: Ken
Retry of the construction of CTLS
Friday, October 15 By: Ken
Screening of CTLS
Cultured CTLS with M9+IPTG. And the propagation couldn't be observed on one sample. We decided to use this sample.
Sequence Analysis
We did the sequencing on ML, MS, CTLS, T4 and CTL. The sequencing of T4 and CTL were in success and the others were failed. The results of CTL was desirable, but that of T4 was bad sequence and there was a mutation on PstI site. Including the last time sequencing, we could say that the deletion of GFP-terminator from SΔTMD1-GFP was failed. So, we decided to retry the deletion PCR.
Sunday, October 17 By: Ken
Retry of deletion PCR of T2
We used PCR to delete GFP and terminator[E0840] from SΔTMD1-E0840.
| Name | Template | 2xBuffer | dNTPs | Primer(fwd) | Primer(rev) | KOD-FX | MilliQ | Total |
|---|---|---|---|---|---|---|---|---|
| GFP-DT-Deletion1, 2 | 0.25 µL | 25 | 10 | 1.5 | 1.5 | 1 | 10.75 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 68℃ | 3.5min | |
| 4℃ | Hold |
After the PCR, digestion by DpnI(37C/60min).
Electrophoresis
- Lane 1,2,5,6: Lambda, 100bp Maker
- Lane 3,4 PCR products[T'1,T'2]
PCR was in success.
Ligation
Ligation PCR products.
Monday, October 18 By: Ken
Transformation
Transformed T'1 and T'2.
| Name | Colony |
|---|---|
| T'1 | Some colonies |
| T'2 | Some colonies |
Thursday, October 21 By: Ken
Making Culture
Cultured two samples on each plate, T'1 and T'2. Named dele1-4. And also cultured lac-SRRz.
Friday, Ocotber 22 By: Ken
Miniprep
| Name | Concentration |
|---|---|
| dele1 | 117.9 ng/µL |
| dele2 | 130.8 |
| dele3 | 108.7 |
| dele4 | 176.0 |
| lac-SRRz | 67.2 |
Saturday, October 23 By: Ken
Sequence Analysis
We used VF2 on dele1-4 and lac-SRRz and VR on lac-SRRz. The sequencing of lac-SRRz with VR was failed, the others were in success. The results were desirable on dele1,3,4 and lac-SRRz.
Deletion PCR of lac-SRRz
We used deletion PCR to get lac-SΔTMD1RRz.
| Name | Template | 2xBuffer | dNTPs | Primer1 | Primer2 | KOD | MilliQ | Total |
|---|---|---|---|---|---|---|---|---|
| lac-SΔTMD1RRz (1) | 0.8 | 25 | 10 | 1.5 | 1.5 | 1 | 10.2 | 50 |
| lac-SΔTMD1RRz (2) | 0.8 | 25 | 10 | 1.5 | 1.5 | 1 | 10.2 | 50 |
| control | 0.8 | 25 | 10 | 1.5 | 1.5 | 0 | 11.2 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 68℃ | 5min | |
| 4℃ | Hold |
After the PCR, done restriction digestion by DpnI(37℃/60min).
Electrophoresis
- Lane 1,8,2,7: Lambda, 100bp Maker
- Lane3,4: lac-SΔTMD1RRz (1,2)
- Lane5: control
Ligation and Transformation
| Name | Colony |
|---|---|
| lac-SΔTMD1RRz(1) | some colonies |
| lac-SΔTMD1RRz(2) | some colonies |
| control | no colony |
Used KRX.
Sunday, October 24 By: Ken
Making Culture
Cultured CTLS, Lysisbox and three samples of lac-SΔTMD1RRz with LB-Tc(IPTG 0 or 1mM).
Monday, October 25 By: Ken
Miniprep
| Name | Concentration |
|---|---|
| CTLS | 62.9 ng/µL |
| Lysisbox | 31.9 |
| lac-SΔTMD1RRz 1 | 140.9 |
| lac-SΔTMD1RRz 2 | 71.6 |
The propagation was not observed on lac-SΔTMD1RRz 3 with LB (IPTG 1mM). It meant this sample might be lac-SRRz. We decided not to use this.
PCR
We used PCR on CTLS and Lysisbox to change its own constitutive promoter to another.
| Name | Template | 2xBuffer | dNTPs | Primer1 | Primer2 | Primer3 | Primer4 | KOD-FX | MilliQ | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| C'TLS(1) | 0.8 µL | 25 | 10 | 1.5 | 1.5 | - | - | 1 | 10.2 | 50 |
| C'TLS(2) | 0.8 µL | 25 | 10 | 1.5 | 1.5 | - | - | 1 | 10.2 | 50 |
| Lysisbox'(1) | 1.5 µL | 25 | 10 | - | - | 1.5 | 1.5 | 1 | 9.5 | 50 |
| Lysisbox'(2) | 1.5 µL | 25 | 10 | - | - | 1.5 | 1.5 | 1 | 9.5 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 68℃ | 6min 20s | |
| 4℃ | Hold |
Primer
- aggtacagtgctagctactagaggagc
- aggactgagctagccgtcaactc
- aggtattatgctagctactagaggagc
- aggactgagctagctgtaaactctag
Electrophoresis
- Lane 1,8,2,7: Lambda, 100bp Maker
- Lane 3,4: C'TLS(1,2)
- Lane 5,6: Lysisbox'(1,2)
Two bands were observed on lane 5 and 6. We decided to do gel extraction.
Ligation
We did ligation of C'TLS(1,2).
Tuesday, October 26 By: Ken
Gel Extraction of Lysisbox'
| Name | Concentration |
|---|---|
| Lysisbox'(1) | 33.6 ng/µL |
| Lysisbox'(2) | 43.4 ng/µL |
Ligation
We did ligation of Lysisbox'(1,2).
Transformaiton
Transformed C'TLS(1,2) and Lysisbox'(1,2).
 "
"