Team:HokkaidoU Japan/Notebook/September23
From 2010.igem.org
(Difference between revisions)
| (7 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
<div class="linkbar"><div class="prev">[[Team:HokkaidoU_Japan/Notebook/September22|September 22]]</div>[[Team:HokkaidoU_Japan/Notebook|Notebook]]<div class="next">[[Team:HokkaidoU_Japan/Notebook/September24|September 24]]</div></div> | <div class="linkbar"><div class="prev">[[Team:HokkaidoU_Japan/Notebook/September22|September 22]]</div>[[Team:HokkaidoU_Japan/Notebook|Notebook]]<div class="next">[[Team:HokkaidoU_Japan/Notebook/September24|September 24]]</div></div> | ||
| - | *Amplifiable BAC plasmid([http://partsregistry.org/Part:BBa_J61031 BBa_J61031]) | + | *Amplifiable BAC plasmid ([http://partsregistry.org/Part:BBa_J61031 BBa_J61031]) purification |
*Colony PCR of AraC+RBS+pSB1A3 | *Colony PCR of AraC+RBS+pSB1A3 | ||
| - | * | + | *Electroporetion of BAC plasmid into DH5α MG1655 |
| - | + | = Bac Vecter Purification = | |
| - | + | Used Qiagen miniprep kit, qiaprep for miniprep | |
| - | 1 | + | #Transfered 1.3 mL of BAC plasmid solution incubated overnight into 1.5 mL tube |
| + | #Centrifuged at 4C, 15000 rpm for 1 min | ||
| + | #Discarded the supernatant and added remaining solution. | ||
| + | #Centrifuged at 4C, 15000 rpm for 1 min | ||
| + | #Discarded the supernatant | ||
| + | #Suspended on 250 uL of Buffer P1 | ||
| + | #Added 250 ul Buffer P2 inverted few times to mix, solution turned green | ||
| + | #Added 350 ul Buffer N3 mixed by inversion, precipitation apeared | ||
| + | #Centrifuged at 4C, 13000 rpm for 10 min | ||
| + | #Transfered the supernatant to filtration column | ||
| + | #Centrifuged at 4C, 13000 rpm for 1 min | ||
| + | #Discarded the flow-through | ||
| + | #added 500 uL of Buffer PB to filtration column | ||
| + | #Centrifuged at 4C, 13000 rpm for 1 min | ||
| + | #Discarded the flow-through centrifuged for 1min to remove remaining buffer | ||
| + | #Transfered filtration column to a new 1.5 ml tube | ||
| + | #Resuspended on 50 ul of TE and incubated at RT for 1min | ||
| + | #Centrifuged at 4C, 13000 rpm for 1 min | ||
| + | #Stored at -20C | ||
| - | |||
| - | + | = Colony PCR of AraC+RBS+pSB1A3 = | |
| - | + | *Selected 16 colonies at random, and PCRed them. | |
| - | + | *PCR mix used is shown in the table bellow | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
{|style="text-align:center; margin-left:100px;" class="protocol" | {|style="text-align:center; margin-left:100px;" class="protocol" | ||
| Line 50: | Line 40: | ||
|- | |- | ||
|Taq Master Mix | |Taq Master Mix | ||
| - | | | + | |80 uL |
|- | |- | ||
|Ex-F | |Ex-F | ||
| - | |1. | + | |1.6 uL |
|- | |- | ||
|Ps-R | |Ps-R | ||
| - | |1. | + | |1.6 uL |
|- | |- | ||
|style="border-top:1px solid #996;"|'''Total''' | |style="border-top:1px solid #996;"|'''Total''' | ||
| - | |style="border-top:1px solid #996;"|''' | + | |style="border-top:1px solid #996;"|'''83.2 uL''' |
|} | |} | ||
| + | |||
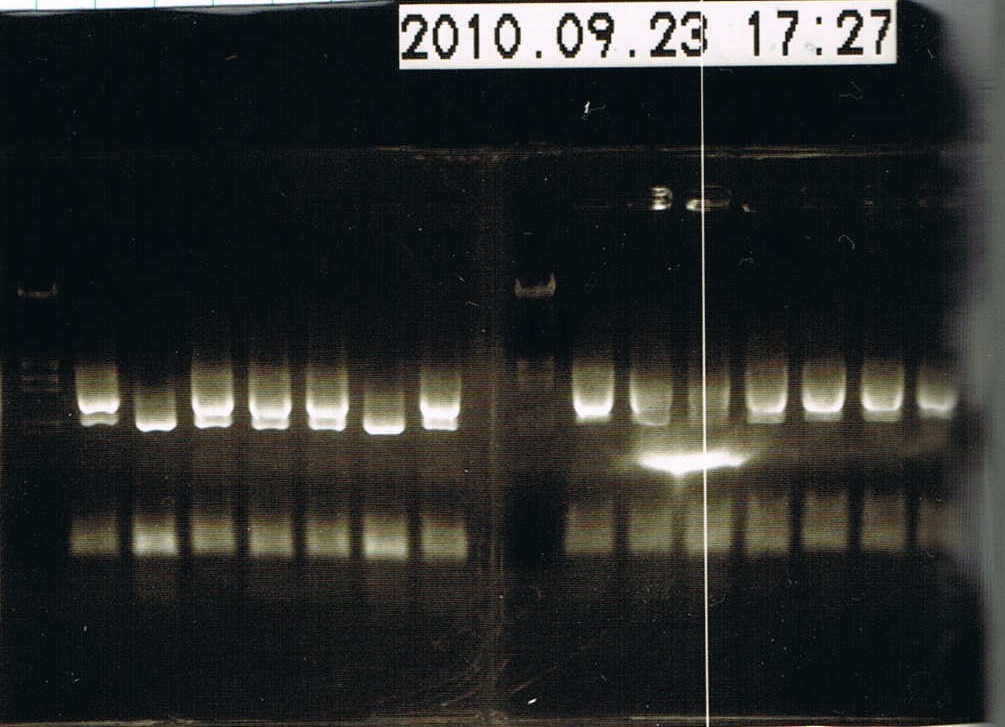
| + | [[Image:9.23 coloP.JPG|200px|right|thumb|Result of colony PCR]] | ||
| + | |||
| + | *electrophoresed the samples | ||
| + | |||
| + | 1 lane -> Marker λ/HindIII EcoRI 5 uL | ||
| + | |||
| + | 2~8 lane -> colony No.1~7 | ||
| + | |||
| + | 9 lane -> Marker λ/HindIII EcoRI 5 uL | ||
| + | |||
| + | 10~16 lane -> colony No.8~14 | ||
| + | |||
| + | |||
| + | Colony No.15,16 didn't electrophorese. | ||
| + | |||
| + | |||
| + | *Colony No.1 will have the plasmid,so we put the sample into 2 mL LB added 2 uL Ampicillin(100 ug/uL).Incubated it in a shaker at 37C, 180 rpm. | ||
Latest revision as of 18:02, 27 October 2010
- Amplifiable BAC plasmid ([http://partsregistry.org/Part:BBa_J61031 BBa_J61031]) purification
- Colony PCR of AraC+RBS+pSB1A3
- Electroporetion of BAC plasmid into DH5α MG1655
Bac Vecter Purification
Used Qiagen miniprep kit, qiaprep for miniprep
- Transfered 1.3 mL of BAC plasmid solution incubated overnight into 1.5 mL tube
- Centrifuged at 4C, 15000 rpm for 1 min
- Discarded the supernatant and added remaining solution.
- Centrifuged at 4C, 15000 rpm for 1 min
- Discarded the supernatant
- Suspended on 250 uL of Buffer P1
- Added 250 ul Buffer P2 inverted few times to mix, solution turned green
- Added 350 ul Buffer N3 mixed by inversion, precipitation apeared
- Centrifuged at 4C, 13000 rpm for 10 min
- Transfered the supernatant to filtration column
- Centrifuged at 4C, 13000 rpm for 1 min
- Discarded the flow-through
- added 500 uL of Buffer PB to filtration column
- Centrifuged at 4C, 13000 rpm for 1 min
- Discarded the flow-through centrifuged for 1min to remove remaining buffer
- Transfered filtration column to a new 1.5 ml tube
- Resuspended on 50 ul of TE and incubated at RT for 1min
- Centrifuged at 4C, 13000 rpm for 1 min
- Stored at -20C
Colony PCR of AraC+RBS+pSB1A3
- Selected 16 colonies at random, and PCRed them.
- PCR mix used is shown in the table bellow
| Reagent | Amount |
|---|---|
| Taq Master Mix | 80 uL |
| Ex-F | 1.6 uL |
| Ps-R | 1.6 uL |
| Total | 83.2 uL |
- electrophoresed the samples
1 lane -> Marker λ/HindIII EcoRI 5 uL
2~8 lane -> colony No.1~7
9 lane -> Marker λ/HindIII EcoRI 5 uL
10~16 lane -> colony No.8~14
Colony No.15,16 didn't electrophorese.
- Colony No.1 will have the plasmid,so we put the sample into 2 mL LB added 2 uL Ampicillin(100 ug/uL).Incubated it in a shaker at 37C, 180 rpm.
 "
"