Team:Groningen/26 July 2010
From 2010.igem.org
| (15 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Groningen/Header}} | {{Team:Groningen/Header}} | ||
| + | |||
| + | |||
'''Week 30''' | '''Week 30''' | ||
| + | ---- | ||
'''Jorrit''' | '''Jorrit''' | ||
| - | Tested | + | Tested expression of week 29 on SDS-page with silver staining on the CFE, medium & pellet samples, results were inconclusive. |
| - | Transformation ''B sub.'' | + | Transformation ''B sub.'' (according to [https://2010.igem.org/Team:Groningen/Protocols_for_Bacillus_subtilis_168 protocol]) |
Inoculated NZ8900 ΔTasA in 10 ml MM, overnight @ 37°C shaker. | Inoculated NZ8900 ΔTasA in 10 ml MM, overnight @ 37°C shaker. | ||
| Line 20: | Line 23: | ||
Plated on LB-agar with antibiotic Cm5/Spec100 and grown @ 37°C (colonies @ 30/7). | Plated on LB-agar with antibiotic Cm5/Spec100 and grown @ 37°C (colonies @ 30/7). | ||
| - | |||
| Line 27: | Line 29: | ||
Two -80°C stocks were made of ''B. sub'' NZ8900 ΔTasA with pNZ8901_E and B. sub NZ8900 ΔTasA with pNZ8901_H. | Two -80°C stocks were made of ''B. sub'' NZ8900 ΔTasA with pNZ8901_E and B. sub NZ8900 ΔTasA with pNZ8901_H. | ||
| + | |||
| + | Chaplin expression (according to [https://2010.igem.org/Team:Groningen/Chaplins protocol]) | ||
Inoculated 4 Greiner tubes with pNZ9801 E6, E2, H1, H3 in 5 ml TY with antibiotic Km5 and Cm5. | Inoculated 4 Greiner tubes with pNZ9801 E6, E2, H1, H3 in 5 ml TY with antibiotic Km5 and Cm5. | ||
| Line 35: | Line 39: | ||
Of all measurementperiodes were taken 2 ml samples, spinned down and put supernatant and cell pellet in freezer. | Of all measurementperiodes were taken 2 ml samples, spinned down and put supernatant and cell pellet in freezer. | ||
| + | |||
| + | |||
| + | '''Modellers:''': | ||
| + | |||
| + | We read and looked up some more articles on biofilm formation models. We found that Professor Van Loosdrecht in Delft knows a lot about this and planned a meeting with him. ''Joël, Arend, Laura, Djoke'' | ||
| + | |||
| + | |||
| + | '''Arend Jan''' | ||
| + | |||
| + | |||
| + | The PCR product from the insertion of Biobrick sites into the pNZ8048 plasmid was cleaned, digested with BamHI, and cleaned again. After cleanup the concentration was to low to continue with a ligation. The cleaned PCR product was used again for a digestion with BamHI and this time the enzyme was then heat inactivated (30min. 80C). To this mixture a 20X ATP solution was added and T4 ligase. After ligation the mixture was transformed to E.coli TOP10. Like before, the plasmid won’t be transformed to E.coli for some reason. The leftover ligation mixture was desalted by use of an osmotic filter and electroporated to Lactococcus lactis (Because the plasmid is originally a lactis plasmid). This also did not work. Should ask if the transformation was done correctly. If so, then go over the cloning strategy again... stupid plasmid. | ||
| + | |||
| + | |||
| + | At the same time we’re still trying to get rid of the IS element. Like before a restriction with MunI and XhoI was performed, this time with both enzymes from Fermentas, on the plasmids pNZ8901-bbs and pNZ8048. | ||
| + | |||
| + | |||
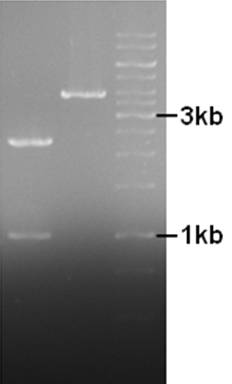
| + | [[Image:28-07-10gn.jpg|150px|thumb|left|pNZ8084 in left lane, pNZ8901-bbs in middle lane.]] | ||
| + | <br style="clear: both" /> | ||
| + | |||
| + | The fragments indicate that XhoI did not cut in the used buffer(G), while it should have 50-100% activity. Not continuing with restrictions but focusing on PCR to get rid of IS element. | ||
| + | '''Geeske''' | ||
| + | Vacation in Berlin, had fun there! | ||
| + | '''David''' | ||
| + | Test purified chaplins | ||
| + | Testing surface tension reduction, by monomerizing 5 mg chaplins via TFA treatment. Then redilute chaplins in 500 ul demiwater an put a drop 50 ul on a petridish and leaf it to dry out overnight. | ||
| + | Next day pipet 50 ul water on to the dried in chaplins and on a clean petri dish and compare. | ||
| + | <br> | ||
| + | [[Image:petrichaplins.jpg]] | ||
{{Team:Groningen/Footer}} | {{Team:Groningen/Footer}} | ||
Latest revision as of 02:30, 28 October 2010
Week 30
Jorrit
Tested expression of week 29 on SDS-page with silver staining on the CFE, medium & pellet samples, results were inconclusive.
Transformation B sub. (according to protocol)
Inoculated NZ8900 ΔTasA in 10 ml MM, overnight @ 37°C shaker.
1 ml overnight culture NZ8900 ΔTasA in 10 ml fresh MM for 3 hours @ 37°C shaker.
Added approx. 1 µg plasmid E and H in two 2 ml tubes and incubated 30 min @ 37°C shaker.
Diluted in pre warmed TY (300 µl) and put for 45 min @ 37°C shaker.
Plated on LB-agar with antibiotic Cm5/Spec100 and grown @ 37°C (colonies @ 30/7).
Inoculated 4 H colonies and 4 E colonies in 5 ml TY with antibiotics Km5 ,Cm5 and Spec100 and put overnight @ 37°C shaker.
Two -80°C stocks were made of B. sub NZ8900 ΔTasA with pNZ8901_E and B. sub NZ8900 ΔTasA with pNZ8901_H.
Chaplin expression (according to protocol)
Inoculated 4 Greiner tubes with pNZ9801 E6, E2, H1, H3 in 5 ml TY with antibiotic Km5 and Cm5.
10 µl of OD cultures (pNZ9801 E6, E2, H1, H3) was taken and grown , overnight @ 37°C shaker, in 5 ml TY with antibiotic Cm5/Spec100 and Cm5.
Done growth curve with OD- measurement.
Of all measurementperiodes were taken 2 ml samples, spinned down and put supernatant and cell pellet in freezer.
Modellers::
We read and looked up some more articles on biofilm formation models. We found that Professor Van Loosdrecht in Delft knows a lot about this and planned a meeting with him. Joël, Arend, Laura, Djoke
Arend Jan
The PCR product from the insertion of Biobrick sites into the pNZ8048 plasmid was cleaned, digested with BamHI, and cleaned again. After cleanup the concentration was to low to continue with a ligation. The cleaned PCR product was used again for a digestion with BamHI and this time the enzyme was then heat inactivated (30min. 80C). To this mixture a 20X ATP solution was added and T4 ligase. After ligation the mixture was transformed to E.coli TOP10. Like before, the plasmid won’t be transformed to E.coli for some reason. The leftover ligation mixture was desalted by use of an osmotic filter and electroporated to Lactococcus lactis (Because the plasmid is originally a lactis plasmid). This also did not work. Should ask if the transformation was done correctly. If so, then go over the cloning strategy again... stupid plasmid.
At the same time we’re still trying to get rid of the IS element. Like before a restriction with MunI and XhoI was performed, this time with both enzymes from Fermentas, on the plasmids pNZ8901-bbs and pNZ8048.
The fragments indicate that XhoI did not cut in the used buffer(G), while it should have 50-100% activity. Not continuing with restrictions but focusing on PCR to get rid of IS element.
Geeske
Vacation in Berlin, had fun there!
David
Test purified chaplins
Testing surface tension reduction, by monomerizing 5 mg chaplins via TFA treatment. Then redilute chaplins in 500 ul demiwater an put a drop 50 ul on a petridish and leaf it to dry out overnight.
Next day pipet 50 ul water on to the dried in chaplins and on a clean petri dish and compare.
File:Petrichaplins.jpg
 "
"