Team:Stockholm/7 October 2010
From 2010.igem.org
(→Andreas) |
m |
||
| (6 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Stockholm/Top2}} | {{Stockholm/Top2}} | ||
| + | |||
==Andreas== | ==Andreas== | ||
| Line 12: | Line 13: | ||
*'''pEX.nLMWP⋅SH.Ry_pEXf:''' ASB0045 772 | *'''pEX.nLMWP⋅SH.Ry_pEXf:''' ASB0045 772 | ||
*'''pEX.nLMWP⋅SH.Ry_pEXf:''' ASB0045 773 | *'''pEX.nLMWP⋅SH.Ry_pEXf:''' ASB0045 773 | ||
| + | |||
| + | *15 μl plasmid DNA; 1.5 μl primer | ||
| + | |||
| + | {|border="1" cellpadding="1" cellspacing="0" | ||
| + | !colspan="3"|DNA concentration | ||
| + | |- | ||
| + | !Sample | ||
| + | !width="50"|Primer | ||
| + | !width="120"|Label | ||
| + | !width="120"|Sequence code | ||
| + | |- | ||
| + | |pEX.nTra10⋅SOD⋅His.RBS.yCCS | ||
| + | |align="center"|pEXf | ||
| + | |pEX.nTra10⋅SH.Ry_pEXf | ||
| + | |align="center"|ASB0045 768 | ||
| + | |- | ||
| + | |pEX.nTra10⋅SOD⋅His.RBS.yCCS | ||
| + | |align="center"|pEXr | ||
| + | |pEX.nTra10⋅SH.Ry_pEXr | ||
| + | |align="center"|ASB0045 769 | ||
| + | |- | ||
| + | |pEX.nTAT⋅SOD⋅His.RBS.yCCS | ||
| + | |align="center"|pEXf | ||
| + | |pEX.nTAT⋅SH.Ry_pEXf | ||
| + | |align="center"|ASB0045 770 | ||
| + | |- | ||
| + | |pEX.nTAT⋅SOD⋅His.RBS.yCCS | ||
| + | |align="center"|pEXr | ||
| + | |pEX.nTAT⋅SH.Ry_pEXr | ||
| + | |align="center"|ASB0045 771 | ||
| + | |- | ||
| + | |pEX.nLMWP⋅SOD⋅His.RBS.yCCS | ||
| + | |align="center"|pEXf | ||
| + | |pEX.nLMWP⋅SH.Ry_pEXf | ||
| + | |align="center"|ASB0045 772 | ||
| + | |- | ||
| + | |pEX.nLMWP⋅SOD⋅His.RBS.yCCS | ||
| + | |align="center"|pEXr | ||
| + | |pEX.nLMWP⋅SH.Ry_pEXr | ||
| + | |align="center"|ASB0045 773 | ||
| + | |} | ||
===Removal of insertion in BioBrick suffixes=== | ===Removal of insertion in BioBrick suffixes=== | ||
| Line 103: | Line 145: | ||
====Gel extraction==== | ====Gel extraction==== | ||
Loaded remaining 17 μl of each sample on a new 1 % agarose gel. Relevant bands excised by gel extraction and saved in -20 ° for later purification. | Loaded remaining 17 μl of each sample on a new 1 % agarose gel. Relevant bands excised by gel extraction and saved in -20 ° for later purification. | ||
| + | |||
| + | ====ON culture==== | ||
| + | Set ON culture of pSB1C3.SOD for later plasmid prep. | ||
| + | *5 ml LB + Cm 25; 37 °C, 225 rpm | ||
===Colony PCR of nCPP⋅SOD⋅His.RBS.yCCS operons in BL21=== | ===Colony PCR of nCPP⋅SOD⋅His.RBS.yCCS operons in BL21=== | ||
| Line 139: | Line 185: | ||
*BL21, pEX.nTAT⋅SOD⋅His | *BL21, pEX.nTAT⋅SOD⋅His | ||
*BL21, pEX.nLMWP⋅SOD⋅His | *BL21, pEX.nLMWP⋅SOD⋅His | ||
| + | |||
| + | ==Nina== | ||
| + | |||
| + | ===Mini prep=== | ||
| + | |||
| + | I performed a mini prep according to the procedure described in protocols. | ||
| + | |||
| + | The experiment was carried out on colony # 1 from protein A _LMWP_N, _TAT_N, _Tra10_N, Fusion _CPP1, _TAT_C, _CPP3, Fusion_LMWP_N, _TAT_N & _Tra10_N | ||
| + | |||
| + | ===Protein induction=== | ||
| + | |||
| + | I prepared a culture of SOD.His (both N & C terminal), SOD_TAT_N, SOD_LMWP_N & SOD_Tra_10 to reach OD600 = 0.6. Then I induced these with IPTG 10 ul/sample. | ||
| + | |||
| + | I took samples at 0, 1, 2 & 3 h, centrifuged 1 min 13000 rpm, resuspended the pellet with 50 ul water and added 50 ul RDSB (with DTT) and stored in a freezer until suitable for loading into an SDS-gel. | ||
| + | |||
| + | ===Making solution=== | ||
| + | |||
| + | I made some solutions of RDSB to have in the freezer when loading protein induction on gel. | ||
| + | |||
| + | Werners sample buffer: | ||
| + | *SDS 4.5 g | ||
| + | *Glycerol 15 % 7.5 ml | ||
| + | *Tris pH 7.8 (30 mM) 1.5 ml (1M) | ||
| + | *Bromophenol Blue (a tip of a spoon) | ||
| + | *H2O up to 50 ml | ||
| + | |||
| + | |||
| + | 900 ul Werners sample buffer + 100 ul DTT (1M) | ||
| + | |||
| + | ===Verification of ligation=== | ||
| + | |||
| + | I ran an agarose gel 1 % 100 V to check if the ligation of IgG_Tra10_N in the pEX vector was successful, but when I looked at the gel there were no bands at all. I must have missed to add the vector and the gene in the ligation. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Mimmi (At Biochemistry) == | ||
| + | |||
| + | === Over expression === | ||
| + | |||
| + | *'''Samples''' | ||
| + | **1ml culture | ||
| + | **centrifuge at 13,000rpm for 1min | ||
| + | **remove supernatant | ||
| + | **resuspend pellet in 50µl H<sub>2</sub>O | ||
| + | **add 50µl sample buffer | ||
| + | **sonicate sample (? for 30s) | ||
| + | **heat sample at 95°C for 5min | ||
| + | **centrifuge sample and load supernatant | ||
| + | |||
| + | *'''Western blot/Comassie 3 gels''' | ||
| + | *''SDS-Page 15%'' | ||
| + | **Acrylamide 15ml | ||
| + | **Resolving buffer 7.5ml | ||
| + | **H<sub>2</sub>O 7.5ml | ||
| + | **SDS 20% 150µl | ||
| + | **TEMED 15µl | ||
| + | **10% amps 90µl | ||
| + | |||
| + | *''Stacker'' | ||
| + | **Acrylamide 670µl | ||
| + | **Stacking buffer 1250µl | ||
| + | **H<sub>2</sub>O 3ml | ||
| + | **SDS 20% 25µl | ||
| + | **TEMED 10µl | ||
| + | **10% amps 37µl | ||
| + | |||
| + | =Johan= | ||
| + | |||
| + | ==Miniprep== | ||
| + | |||
| + | tra-bFGF-his, tra-bFGF-his, lmwp-bFGF-his, tat-bFGF-his, his-bFGF-tra10, his-bFGF-tat, his-bFGF-lmwp | ||
| + | |||
| + | ~250ng/µl | ||
| + | |||
| + | ==Cut miniprep== | ||
| + | |||
| + | 2 µl DNA | ||
| + | (1 µl BamHI) | ||
| + | 2 µl 10x fastbuffer | ||
| + | 15 µl H2O | ||
| + | |||
| + | 30 min 37 °C | ||
| + | |||
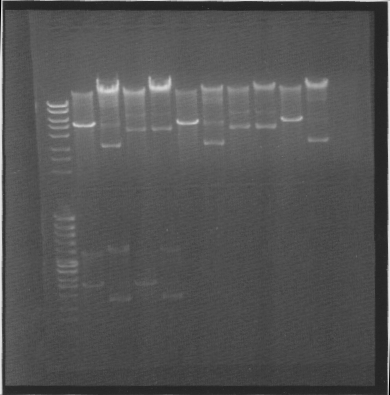
| + | ==Gel== | ||
| + | |||
| + | Of cut and uncut minipreps | ||
| + | |||
| + | [[Image:SU 7oktgels 1.png]] | ||
| + | |||
| + | Results: The second tra10-bFGF-his dont work, I took two colonies from that construct for a reason :). tat-bFGF-his dont work, I have to re-do that construct. | ||
| + | |||
| + | ==Cut his-bFGF & bFGF-his== | ||
| + | |||
| + | To put into pEX. | ||
| + | |||
| + | 5 µl his-bFGF & bFGF-his | ||
| + | |||
| + | 2 µl 10x fastbuffer | ||
| + | |||
| + | 1 µl XbaI | ||
| + | |||
| + | 1 µl PstI | ||
| + | |||
| + | 11 µl H2O | ||
| + | |||
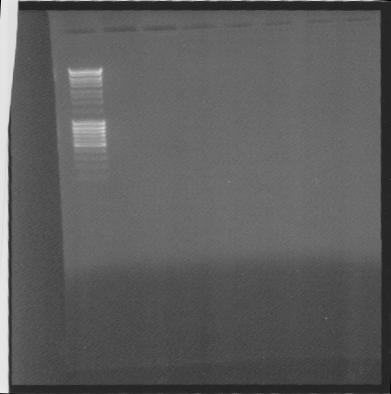
| + | ==Gel of Ninas samples== | ||
| + | |||
| + | I had time over so I did a gel of | ||
| + | * tra10-igg-his ligated into pEX, to see if the gene is ligated | ||
| + | * 10 samples of another constructs | ||
| + | |||
| + | [[Image:SU 7oktgels.png]] | ||
| + | |||
| + | Results: Noting.. Nina must have done something wrong.. | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Latest revision as of 20:05, 27 October 2010
Contents |
Andreas
Transfer of nCPP⋅SOD⋅His.RBS.yCCS operons to pEX
Sequencing
15 μl plasmid DNA, 1.5 μl primer
- pEX.nTra10⋅SH.Ry_pEXf: ASB0045 768
- pEX.nTra10⋅SH.Ry_pEXr: ASB0045 769
- pEX.nTAT⋅SH.Ry_pEXf: ASB0045 770
- pEX.nTAT⋅SH.Ry_pEXf: ASB0045 771
- pEX.nLMWP⋅SH.Ry_pEXf: ASB0045 772
- pEX.nLMWP⋅SH.Ry_pEXf: ASB0045 773
- 15 μl plasmid DNA; 1.5 μl primer
| DNA concentration | |||
|---|---|---|---|
| Sample | Primer | Label | Sequence code |
| pEX.nTra10⋅SOD⋅His.RBS.yCCS | pEXf | pEX.nTra10⋅SH.Ry_pEXf | ASB0045 768 |
| pEX.nTra10⋅SOD⋅His.RBS.yCCS | pEXr | pEX.nTra10⋅SH.Ry_pEXr | ASB0045 769 |
| pEX.nTAT⋅SOD⋅His.RBS.yCCS | pEXf | pEX.nTAT⋅SH.Ry_pEXf | ASB0045 770 |
| pEX.nTAT⋅SOD⋅His.RBS.yCCS | pEXr | pEX.nTAT⋅SH.Ry_pEXr | ASB0045 771 |
| pEX.nLMWP⋅SOD⋅His.RBS.yCCS | pEXf | pEX.nLMWP⋅SH.Ry_pEXf | ASB0045 772 |
| pEX.nLMWP⋅SOD⋅His.RBS.yCCS | pEXr | pEX.nLMWP⋅SH.Ry_pEXr | ASB0045 773 |
Removal of insertion in BioBrick suffixes
An insertion between SpeI and PstI present in IgGp, bFGF, ProtA, yCCS and SOD needs to be removed before submission to the Registry. This will be done by digestion with SpeI (inside insertion) and moving digested gene into a new vector.
Digestions
No sample of SOD available for the moment, so this will be digested after plasmid prep.
| pC.IgGp (A) | pC.bFGF (B) | pC.ProtA (C) | pC.yCCS (D) | pC.RFP (E) | |
|---|---|---|---|---|---|
| 10X FastDigest buffer | 2 | 2 | 2 | 2 | 2 |
| DNA | 6 | 10 | 16 | 14 | 11 |
| dH2O | 10 | 5 | 0 | 2 | 5 |
| FD SpeI | 1 | 1 | 1 | 1 | 1 |
| FD EcoRI | 1 | 1 | 1 | 1 | 1 |
| 20 μl | 20 μl | 20 μl | 20 μl | 20 μl |
- Incubation: 37 °C, 30 min
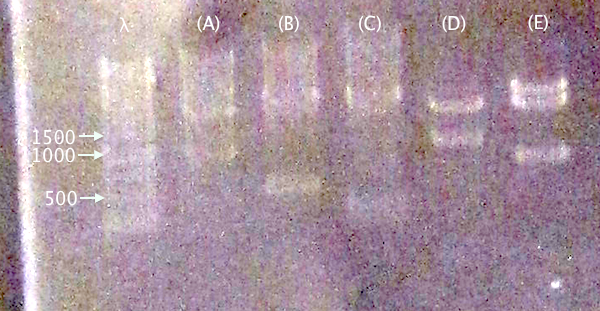
Gel verification
1 % agarose, 130 V
IgGp bFGF ProtA yCCS RFP
Expected bands
- pSB1C3.IgGp (A)
- Vector: 2050 bp
- Insert: 990 bp
- pSB1C3.bFGF (B)
- Vector: 2050 bp
- Insert: 520 bp
- pSB1C3.ProtA (C)
- Vector: 2050 bp
- Insert: 230 bp
- pSB1C3.yCCS (D)
- Vector: 2050 bp
- Insert: 800 bp
- pSB1C3.RFP (E)
- Vector: 2050 bp
- Insert: 1120 bp
Results
Relevant-sized for all samples showing successful (but incomplete) digestion. Proceeded to gel extraction.
Gel extraction
Loaded remaining 17 μl of each sample on a new 1 % agarose gel. Relevant bands excised by gel extraction and saved in -20 ° for later purification.
ON culture
Set ON culture of pSB1C3.SOD for later plasmid prep.
- 5 ml LB + Cm 25; 37 °C, 225 rpm
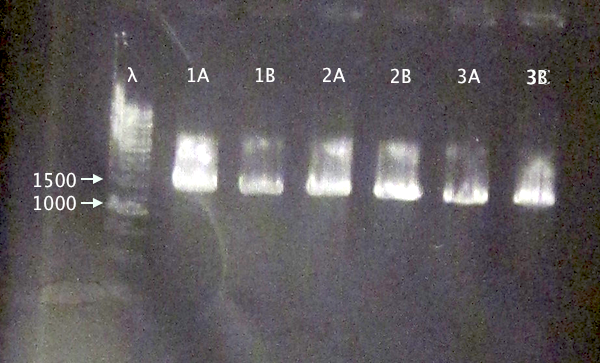
Colony PCR of nCPP⋅SOD⋅His.RBS.yCCS operons in BL21
- BL21 pEX.nTra10⋅SOD⋅His.RBS.yCCS (A & B)
- BL21 pEX.nTAT⋅SOD⋅His.RBS.yCCS (A & B)
- BL21 pEX.nLMWP⋅SOD⋅His.RBS.yCCS (A & B)
- Standard colony PCR settings.
- Elongation: 1:30 (too short?)
Gel verification
1 % agarose, 130 V
Expected bands
- 1553 bp
- 1523 bp
- 1532 bp
Results
Bands with relevant sizes for all clones.
ON cultures
3 ml LB + Amp 100
- B
- B
- B
Growth curve assay
A growth curve assay will be run to test the toxicity of our CPPs.
ON cultures
5 ml LB + Amp 100; 37 °C, 225 rpm
- BL21, pEX.SOD⋅His
- BL21, pEX.nTra10⋅SOD⋅His
- BL21, pEX.nTAT⋅SOD⋅His
- BL21, pEX.nLMWP⋅SOD⋅His
Nina
Mini prep
I performed a mini prep according to the procedure described in protocols.
The experiment was carried out on colony # 1 from protein A _LMWP_N, _TAT_N, _Tra10_N, Fusion _CPP1, _TAT_C, _CPP3, Fusion_LMWP_N, _TAT_N & _Tra10_N
Protein induction
I prepared a culture of SOD.His (both N & C terminal), SOD_TAT_N, SOD_LMWP_N & SOD_Tra_10 to reach OD600 = 0.6. Then I induced these with IPTG 10 ul/sample.
I took samples at 0, 1, 2 & 3 h, centrifuged 1 min 13000 rpm, resuspended the pellet with 50 ul water and added 50 ul RDSB (with DTT) and stored in a freezer until suitable for loading into an SDS-gel.
Making solution
I made some solutions of RDSB to have in the freezer when loading protein induction on gel.
Werners sample buffer:
- SDS 4.5 g
- Glycerol 15 % 7.5 ml
- Tris pH 7.8 (30 mM) 1.5 ml (1M)
- Bromophenol Blue (a tip of a spoon)
- H2O up to 50 ml
900 ul Werners sample buffer + 100 ul DTT (1M)
Verification of ligation
I ran an agarose gel 1 % 100 V to check if the ligation of IgG_Tra10_N in the pEX vector was successful, but when I looked at the gel there were no bands at all. I must have missed to add the vector and the gene in the ligation.
Mimmi (At Biochemistry)
Over expression
- Samples
- 1ml culture
- centrifuge at 13,000rpm for 1min
- remove supernatant
- resuspend pellet in 50µl H2O
- add 50µl sample buffer
- sonicate sample (? for 30s)
- heat sample at 95°C for 5min
- centrifuge sample and load supernatant
- Western blot/Comassie 3 gels
- SDS-Page 15%
- Acrylamide 15ml
- Resolving buffer 7.5ml
- H2O 7.5ml
- SDS 20% 150µl
- TEMED 15µl
- 10% amps 90µl
- Stacker
- Acrylamide 670µl
- Stacking buffer 1250µl
- H2O 3ml
- SDS 20% 25µl
- TEMED 10µl
- 10% amps 37µl
Johan
Miniprep
tra-bFGF-his, tra-bFGF-his, lmwp-bFGF-his, tat-bFGF-his, his-bFGF-tra10, his-bFGF-tat, his-bFGF-lmwp
~250ng/µl
Cut miniprep
2 µl DNA (1 µl BamHI) 2 µl 10x fastbuffer 15 µl H2O
30 min 37 °C
Gel
Of cut and uncut minipreps
Results: The second tra10-bFGF-his dont work, I took two colonies from that construct for a reason :). tat-bFGF-his dont work, I have to re-do that construct.
Cut his-bFGF & bFGF-his
To put into pEX.
5 µl his-bFGF & bFGF-his
2 µl 10x fastbuffer
1 µl XbaI
1 µl PstI
11 µl H2O
Gel of Ninas samples
I had time over so I did a gel of
- tra10-igg-his ligated into pEX, to see if the gene is ligated
- 10 samples of another constructs
Results: Noting.. Nina must have done something wrong..
 |

|
 |

|
 |

|

|

|
 "
"