Team:Groningen/7 June 2010
From 2010.igem.org
(Difference between revisions)
| Line 24: | Line 24: | ||
[[Image:08-06-10gn.jpg|200px|thumb|left|Last time in bad quality, I promise]] | [[Image:08-06-10gn.jpg|200px|thumb|left|Last time in bad quality, I promise]] | ||
| + | |||
| + | <br style="clear: both" /> | ||
| + | |||
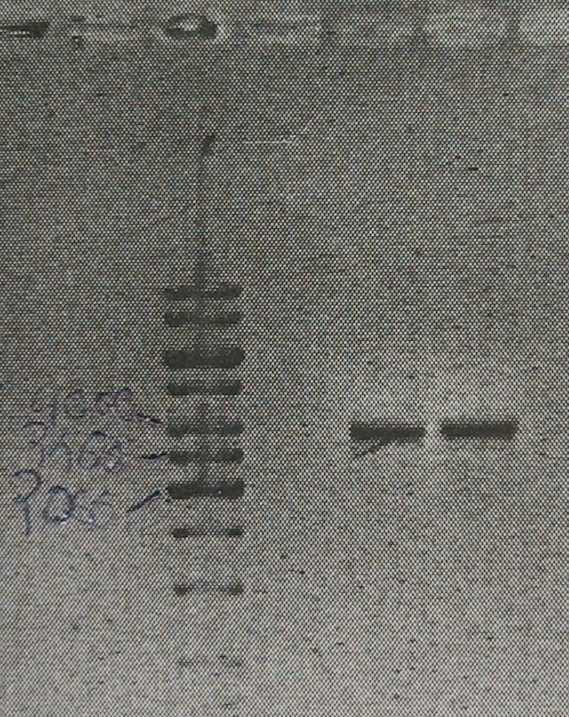
Using 10x pfu buffer + MgSO4 (instead of adding MgCl2) seems to decrease smear. Also, increasing the elongation time to 5min seems to give more product. Product was cleaned (Roche kit)and digested with BamHI and ligated. | Using 10x pfu buffer + MgSO4 (instead of adding MgCl2) seems to decrease smear. Also, increasing the elongation time to 5min seems to give more product. Product was cleaned (Roche kit)and digested with BamHI and ligated. | ||
Revision as of 11:49, 13 October 2010
Week 23, Arend Jan
All the PCR product was used up so a new PCR was performed.
Component µl Final concentration Primer pNZ89bbs-for1 5 300nM Primer pNZ89bbs-rev1 5 300nM 10x pfu buffer(+MgSO4) 5 1x dNTP’s 2 200µM Template 0.5 ~14ng Pfu polymerase 1 2.5u MQ 31.5 - 94°C, 3’ - 94°C, 30’’ 25X - 50°C, 30’’ - 72°C, 5’ - 72°C, 10’
Using 10x pfu buffer + MgSO4 (instead of adding MgCl2) seems to decrease smear. Also, increasing the elongation time to 5min seems to give more product. Product was cleaned (Roche kit)and digested with BamHI and ligated.
- 22ul plasmid - 2.5ul buffer G - 0.5ul BamHI - 10ul/20ul DNA (~100ng/~200ng) - 5ul T4 ligase buffer - 5ul T4 ligase -30ul/20ul MQ
25ul of ligation mixture was used for transforming 100ul compentent cells. Good amount of colonies. Colonies were picked, grown overnight, and minipreped using the High Pure Plasmid Isolation kit from Roche.
Long weekend including Paris workshop.
 "
"