Team:Freiburg Bioware/NoteBook/Labjournal/October
From 2010.igem.org
(→Mini Prep of pGa14_MiddleLinker_VP2/3_inscap and pSB1C3_Darpin) |
(→137. labday 02.10.2010) |
||
| Line 204: | Line 204: | ||
===<p style="font-size:17px; background-color:#00dd77;">137. labday 02.10.2010</p>=== | ===<p style="font-size:17px; background-color:#00dd77;">137. labday 02.10.2010</p>=== | ||
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>BioBrick Assembly of CD and mCherry</b></p>==== | ||
| + | <b>Investigator: Bea</b> | ||
| + | <br/> | ||
| + | <p style="color:#66bbff;"><i>Comment</i>: For assembling the last BioBricks together in order to obtain the fully assembled vectorplasmid which contains the tumorspecific promoter phTERT and the GOI mVenus.</p> | ||
| + | <br /> | ||
| + | <b>Digestion of the constructs:</b> | ||
| + | <ul> | ||
| + | <li>P = pSB1C3_leftITR_phTERT_betaglobin_mVenus c=257 ng/µL</li> | ||
| + | <li>P = pSB1C3_hGH_rightITR c=105 ng/µL</li> | ||
| + | </ul> | ||
| + | <br /> | ||
| + | {| border="1" | ||
| + | | align="left" | '''Components''' ||align="left"| <b>v<sub>P485</sub> /µL</b> ||align="left"| <b>v<sub>P186</sub>/µL</b> | ||
| + | |- | ||
| + | | align="left" | DNA ||align="left"| 4||align="left"| 14 | ||
| + | |- | ||
| + | | align="left" | BSA (10x) ||align="left"| 2||align="left"| 2.5 | ||
| + | |- | ||
| + | | align="left" | Buffer no. 4 (10x) ||align="left"| 2||align="left"| 2.5 | ||
| + | |- | ||
| + | | align="left" | EcoRI ||align="left"| 1||align="left"| 1 | ||
| + | |- | ||
| + | | align="left" | AgeI ||align="left"| 1||align="left"| 1 | ||
| + | |- | ||
| + | | align="left" | H<sub>2</sub>O ||align="left"| 10||align="left"| 4 | ||
| + | |- | ||
| + | | align="left" | '''Total volume''' ||align="left"| '''20''' ||align="left"| '''25''' | ||
| + | |- | ||
| + | |} | ||
| + | <br /> | ||
| + | The expected fragments of the digested constructs were: The underlined fragments represent the fragments which correspond to the desired construct which can be used for ligation. | ||
| + | <br /> | ||
| + | <ul> | ||
| + | |||
| + | <li><b>P485:</b> <u>3911bp</u></li> | ||
| + | <li><b>P186:</b> <u>657</u>, 2072bp</li> | ||
| + | </ul> | ||
| + | <br /> | ||
| + | <b>Loading plan: </b> | ||
| + | |||
| + | M P P | ||
| + | <br /> | ||
| + | <b>Results:</b> | ||
| + | [[Image: |thumb|center|400px]] | ||
| + | <br /> | ||
| + | After gel extraction has been performed, the ligation was carried out. | ||
| + | <br /> | ||
| + | <br /> | ||
| + | |||
| + | <b>Ligation:</b> | ||
| + | <ul> | ||
| + | <li>v<sub>=P485 </sub>=4,96µL </li> | ||
| + | <li>v<sub>=P309 </sub>=3,04µL</li> | ||
| + | </ul> | ||
| + | The ligation mix was transformed into XL1-Blue cells and plated on agar plates containing chloramphenicol. | ||
| + | <br /> | ||
| + | <br /> | ||
| + | |||
| + | <b>Next steps:</b> | ||
| + | <br /> | ||
| + | Picking clones and perform Mini-Prep. After the Mini-Preps have been performed this construct needs to be sequenced and tested in cell culture. | ||
| + | |||
| + | |||
====<p style="font-size:15px; background-color:#66bbff;"><b>Mini Prep and test digestion of pGa14_MiddleLinker_VP2/3_HSPG-KO</b></p>==== | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini Prep and test digestion of pGa14_MiddleLinker_VP2/3_HSPG-KO</b></p>==== | ||
'''Investigator: Hanna''' | '''Investigator: Hanna''' | ||
Revision as of 12:09, 2 October 2010
136. labday 01.10.2010
mini preps of CD clones
Investigator: Kira
c(p689)= 115 ng/ul
c(690)= 151, 20 ng/ul
c(691)= 122,27 ng/ul
c(p692) = 126,42 ng/ul
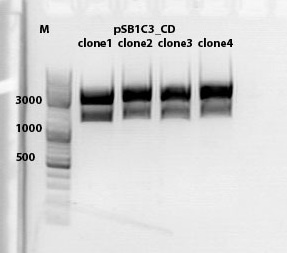
test digestion of CD clones
Investigator: Kira
| Components | sample Volume/µL |
| DNA | 4,0 µl |
| BSA (10x) | 2 µl |
| Buffer no. 4 | 2,0 µl |
| Enzyme 1 XbaI | 1,0 µl |
| Enzyme 2 AgeI | 1,5 µl |
| H2O | 9,5 µl |
| Total volume | 20 |
incubation @ 37 C for approx. 2 h
1% agarose gel
Biobrick assembly pSB1C3_lITR_hTERT_beta-globin_CD
Investigator: Kira
c(pSB1C3_lITR_hTERT_beta-globin)= 333 ng/ul
c(pSB1C3_CD)= 151 ng/ul
| Components | vector Volume/µL | insert Volume/µL |
| DNA | 4,5 µl | 6 |
| BSA (10x) | 3 µl | 3 |
| Buffer no. 4 | 3,0 µl | 3 |
| Enzyme 1 XbaI | 0 µl | 1,5 |
| Enzyme 2 SpeI | 1,5 µl | 0 |
| Enzyme 3 PstI-HF | 1,0 | 1 |
| H2O | 17 | 15,5 |
| Total volume | 25 |
incubation @ 37 C for approx. 2 h
1% agarose gel
Ligation
DNA-mix: 8 ul (vector 4,6ul)+(insert 3,4 ul)
T4 ligase: 1 ul
T4 buffer: 1 ul
Incubation @ RT for 30 min
Transformation was performed according to the standard protocol w BL21 cells.
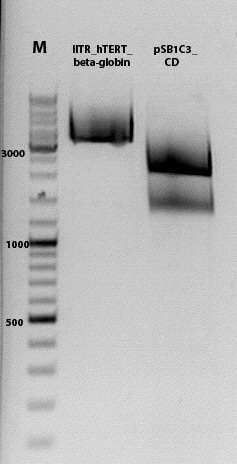
Sequencing results of pCerulean_Zegfr:1907_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Comment: All N-terminal fusion approaches with VP2/3_HSPG-KO revealed positive results except of pCerulean_Zegfr:1907_MiddleLinker_VP2/3_HSPG-KO. Another clone was picked, preped, test digested and sent for sequencing.
Conclusion: Sequencing results revealed positive results.
Picking clones of pGA14_MiddleLinker_VP2/3_insCap and pGA14_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Unfortunately there grew a bacteria lawn over night - it was hardly not possible to pick clones. Nevertheless I tried and picked 2 clones of pGA14_MiddleLinker_VP2/3_insCap and pGA14_MiddleLinker_VP2/3_HSPG-KO.
To do: Mini-Prep and test digestion.
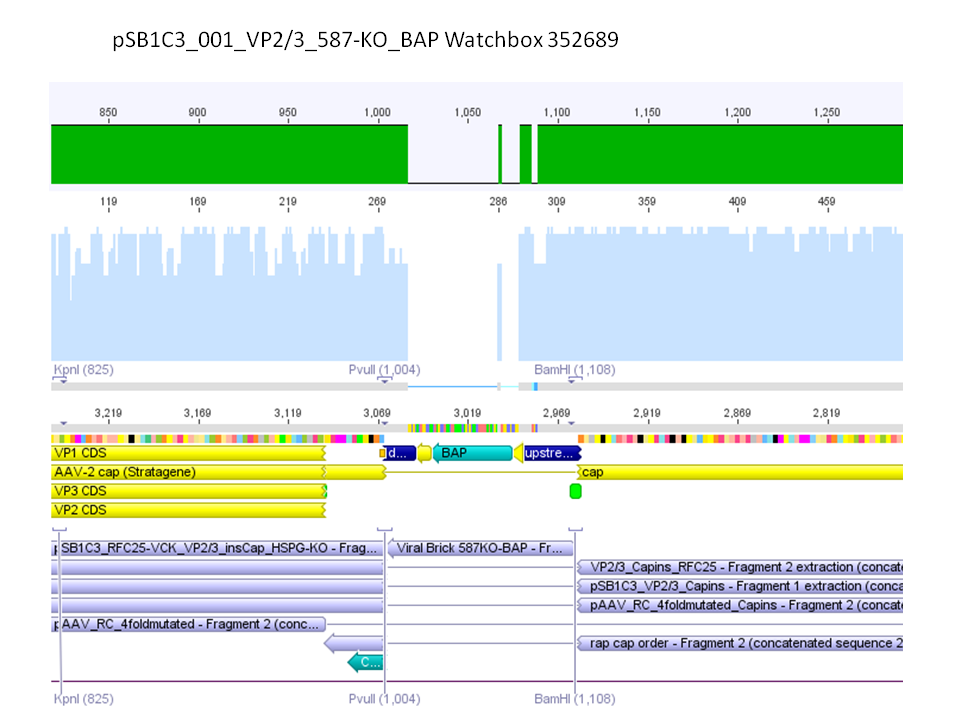
Sequencing results of pSB1C3_001_VP2/3_587-KO_BAP and pSB1C3_001_VP2/3_587-KO_6xHis
Investigator: Hanna
Comment: For the creation of our super constructs, the His-Tag and the BAP motif need to be cloned into VP2/3 for N-terminal fusion to VP2. Sequencing results showed that the 6xHis and BAP motif was not cloned into VP2/3_insCap.
To do: Clone 587-KO_6xHis and 587-KO_BAP into pSB1C3_001_VP2/3_insCap 1. via digestion of inserts and 2. via hybridization of referring oligos - digestion of vector with 800-900 ng.
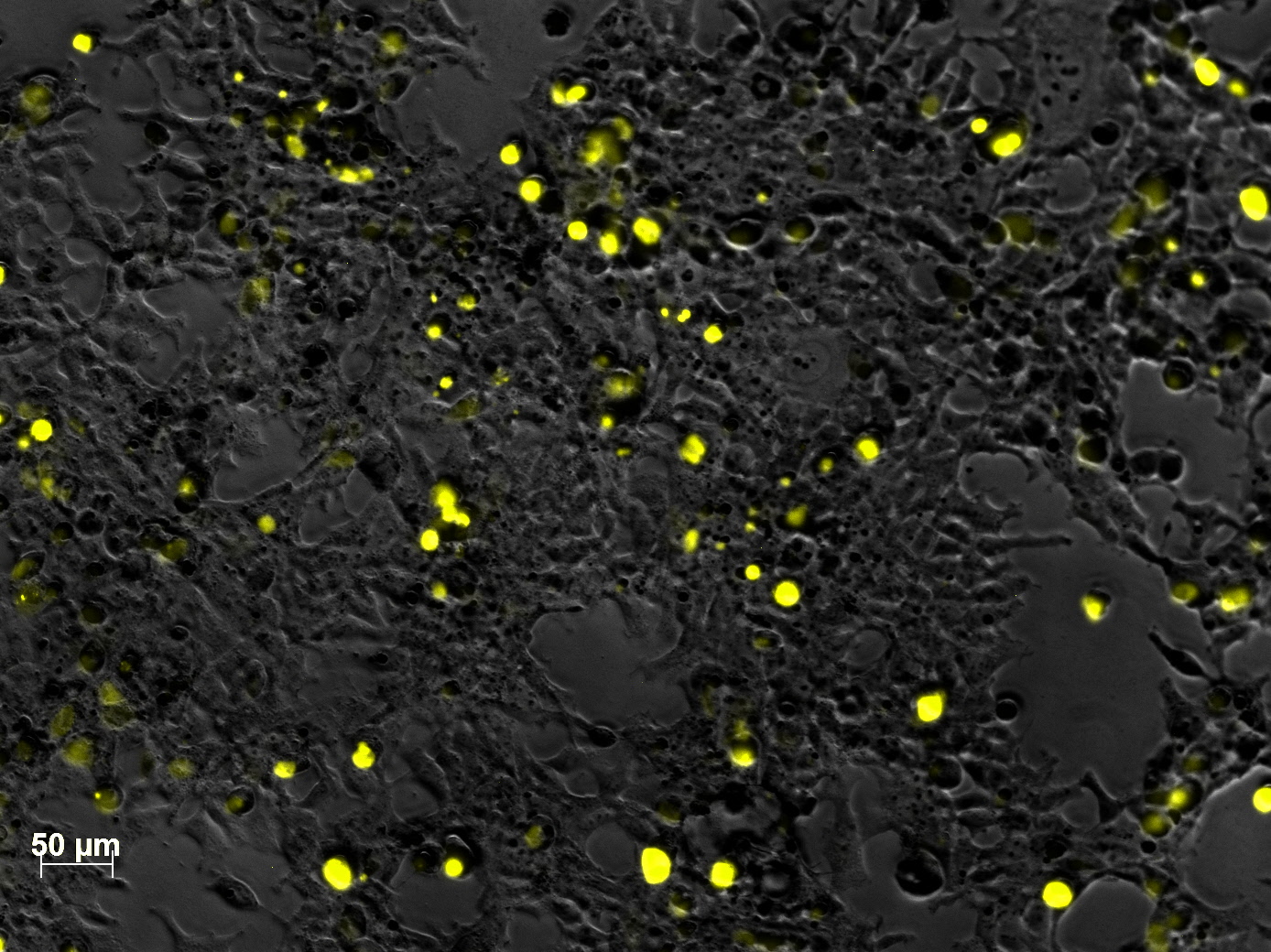
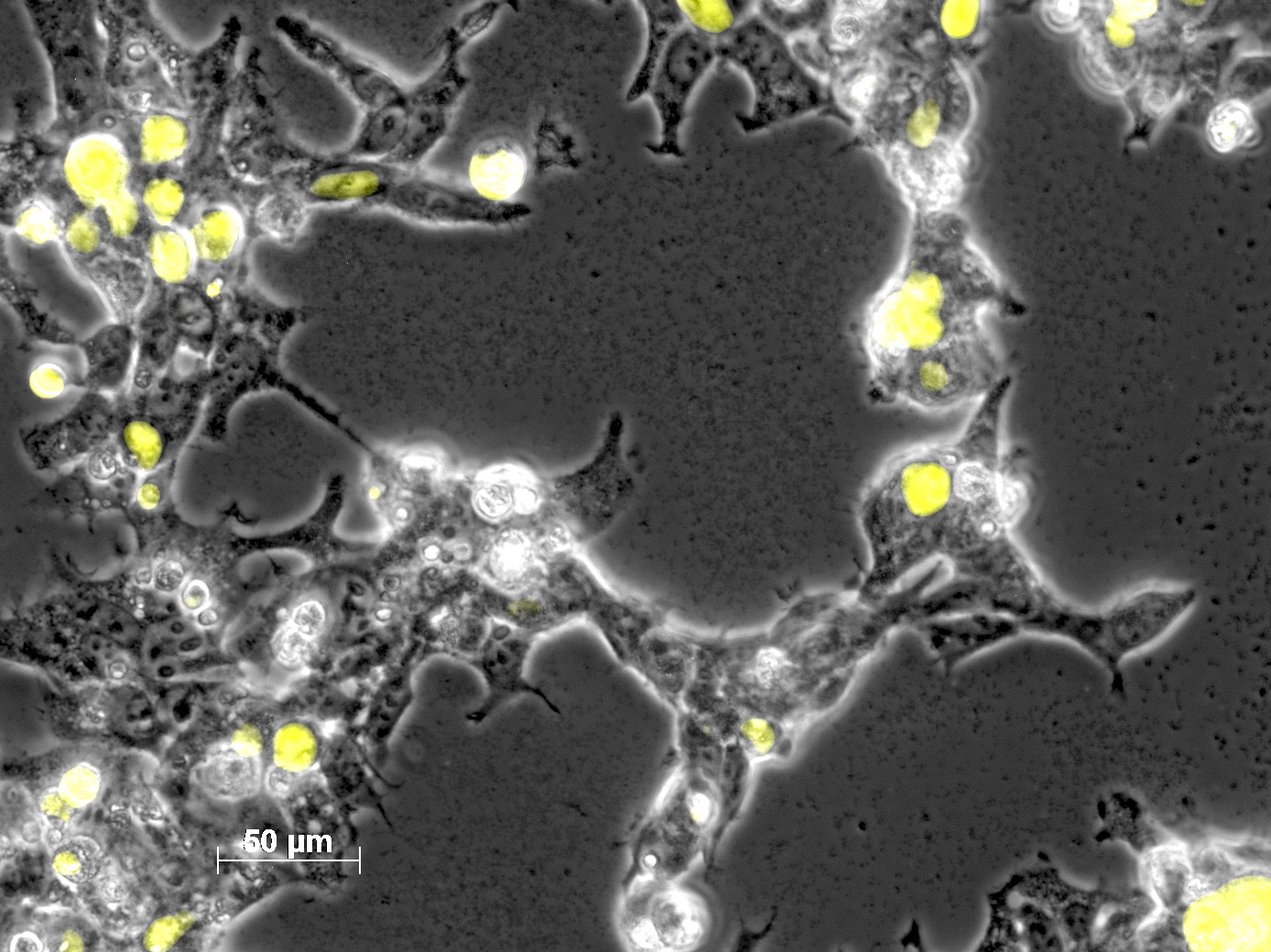
Impressions of transfection of AAV293 with pCerulean_VP1up_NLS_mVenus_VP2/3
Investigator: Adrian
Yesterday AAV293 cells were transfected with pCerulean_VP1up_NLS_mVenus_VP2/3 and GMK-TK30 as gene of interest. Today, nuclear localization of the produced mVenus_VP2/3 fusion protein was visible and can be nicely seen in the following pictures:
Conclusion: The VP2/3 fusion particle was transcribed and translated AND was transported back into the nucleus in order to be packaged by the ITR-flanked gene of interest.
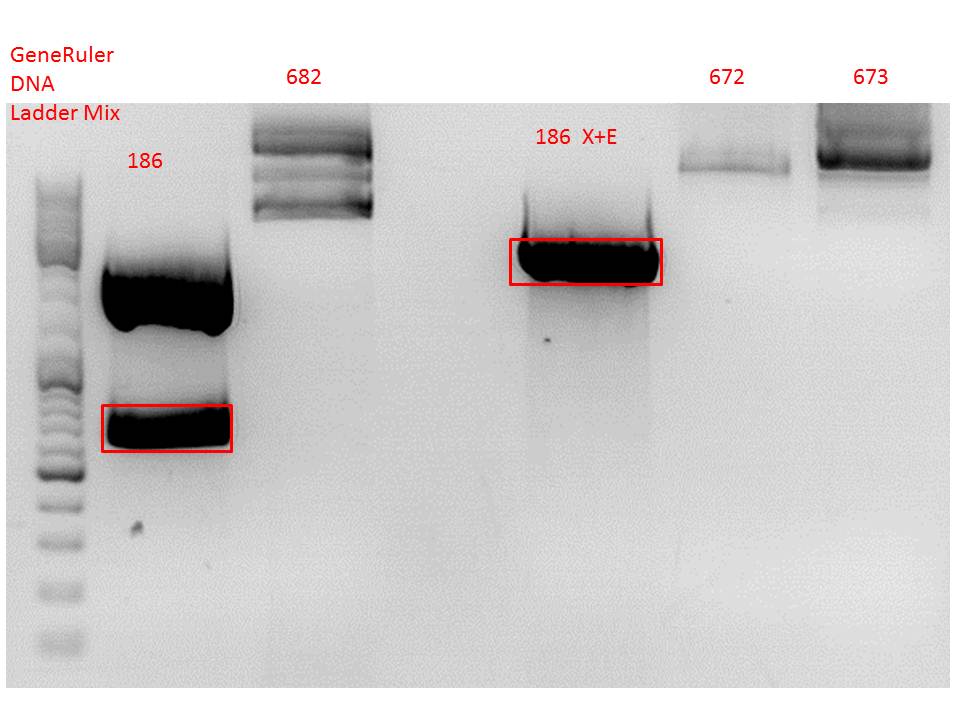
Cloning of hGH_rITR into pSB1C3_lITR_phTERT_beta-globin_CFP and lITR_phTERT_beta-globin_mGMK_TK30 into pSB1C3_hGH_rITR
Investigator: Stefan
Vector name:
- pSB1C3_lITR_phTERT_beta-globin_CFP (P682)
- pSB1C3_hGH_rITR (P186)
Insert name:
- pSB1C3_hGH_rITR (P186)
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P672)
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P673)
| components | volume for P186 /µl | volume of P186 X+E /µl | volume of P672 + P673 /µl | volume of P682 /µl |
| DNA | 12 | 12 | 5 | 14 |
| BSA (10x) | 2,5 | 2 | 2 | 2 |
| Buffer 4 (10x) | 3 | 2 | 2 | 2 |
| Enzyme XbaI | 1 | 1 | - | - |
| Enzyme PstI | 1 | - | - | 1 |
| Enzyme EcoI | - | 1 | 1 | - |
| Enzyme SpeI | - | - | 1 | 1 |
| H2O | 6 | 6 | 9 | - |
| Total volume (e.g. 15,20,25,30 µl) | 25 | 25 | 20 | 20 |
Gel:
0,5 g Agarose, 50 ml TAE (1%), 3 µl GELRED , at 115 Volt
Comment: Since the first gel revealed problems with samples 672, 673 and 682 another digestion was prepared (same approach as shown above), this time using a 0,8% gel and another loading dye (without SDS).
0,4 g Agarose, 50 ml TAE (0,8%), 3 µl GELRED , at 115 Volt
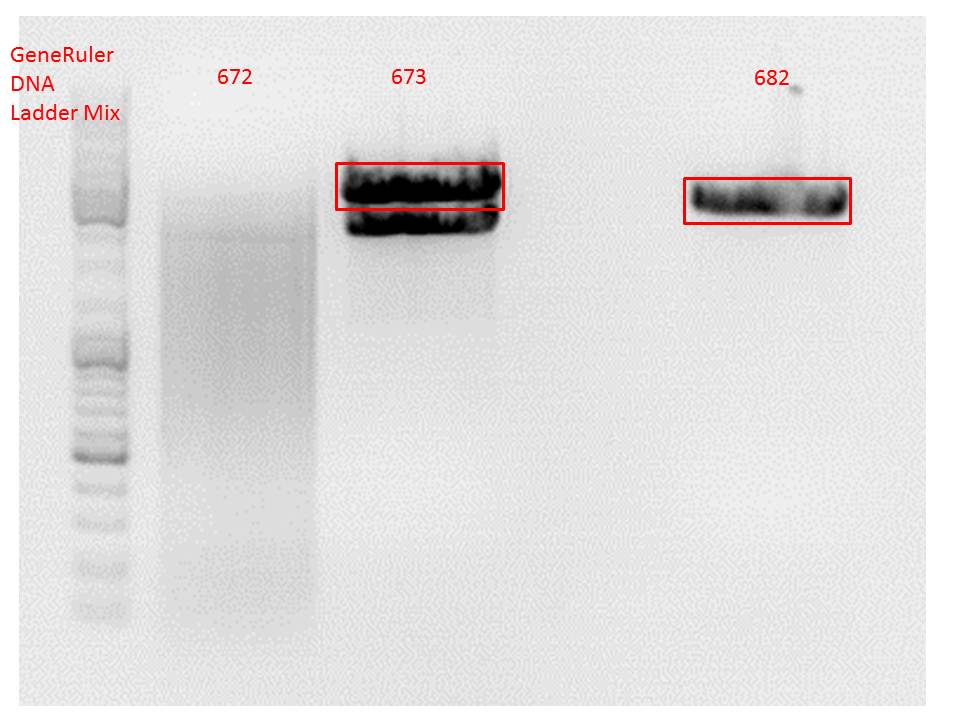
Comment: Since sample 672 showed no bands this sample was discarded and cloning continued only using 673.
Gel extraction:
Was performed according to protocol.
T4 Ligation:
Ligation was performed over night.
| ligation name | 186 (X+E) + 673 | 186 + 682 |
| volume of vector | 3,51 | 4,49 |
| volume of insert | 5,38 | 2,64 |
Transformation:
Will be done tomorrow using BL21 cells.
137. labday 02.10.2010
BioBrick Assembly of CD and mCherry
Investigator: Bea
Comment: For assembling the last BioBricks together in order to obtain the fully assembled vectorplasmid which contains the tumorspecific promoter phTERT and the GOI mVenus.
Digestion of the constructs:
- P = pSB1C3_leftITR_phTERT_betaglobin_mVenus c=257 ng/µL
- P = pSB1C3_hGH_rightITR c=105 ng/µL
| Components | vP485 /µL | vP186/µL |
| DNA | 4 | 14 |
| BSA (10x) | 2 | 2.5 |
| Buffer no. 4 (10x) | 2 | 2.5 |
| EcoRI | 1 | 1 |
| AgeI | 1 | 1 |
| H2O | 10 | 4 |
| Total volume | 20 | 25 |
The expected fragments of the digested constructs were: The underlined fragments represent the fragments which correspond to the desired construct which can be used for ligation.
- P485: 3911bp
- P186: 657, 2072bp
Loading plan:
M P P
Results:
[[Image: |thumb|center|400px]]
After gel extraction has been performed, the ligation was carried out.
Ligation:
- v=P485 =4,96µL
- v=P309 =3,04µL
The ligation mix was transformed into XL1-Blue cells and plated on agar plates containing chloramphenicol.
Next steps:
Picking clones and perform Mini-Prep. After the Mini-Preps have been performed this construct needs to be sequenced and tested in cell culture.
Mini Prep and test digestion of pGa14_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Comment: In order to fuse the DARPin to the N-terminus of VP2/3_insCap and VP2/3_HSPG-KO, these motifs need to be fused to the Middle Linker (performed on 30.9.2010). In order to be able to immediately continue with cloning, BL21 cells were transformed and clones were picked in the noon. Now, the DNA needs to be preped and test digested in order to perform a 3-fragment-ligation with the DARPin and the pSB1C3_001_CMV plasmid. Unfortunately the cells of the VP2/3_insCap construct grew not densely enough. Therefore two new clones were picked from the plate and were inoculated in 10 mL LB medium. The other construct (pGa14_MiddleLinker_VP2/3_HSPG-KO) was preped and test digested.
TEST DIGESTION:
For both clones:
- DNA: 4 µL
- Buffer 4: 1 µL
- BSA: 1 µL
- EcoRI: 0.5 µL
- PstI: 0.5 µL
- H2O: 3 µL
Incubation: 1 hour.
Agarose gel: 0.8 %, 115 Volt, 1 hour.
Comment: The choice of these restriction enzymes was not clever, because if cloning didn't work, I expect one band at 2379 bp (the insert should hardly be seen) - if it worked one band at 2379 bp and a second at 2005 bp. Fortunately the bands could be separated ans revealed positive results for both clones :
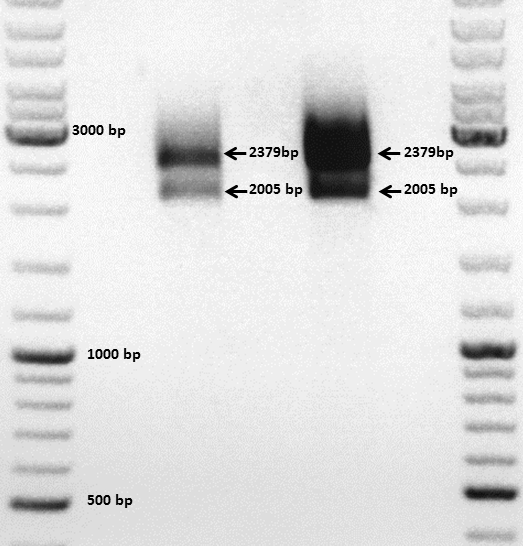
Next steps:
- Mini-Prep and test digestion of pGa14_MiddleLinker_VP2/3_ins Cap.
- 3 fragment ligation of MiddleLinker_VP2/3_ins Cap or MiddleLinker_VP2/3_HSPG-KO + DARPin + pSB1C3_001_CMV backbone.
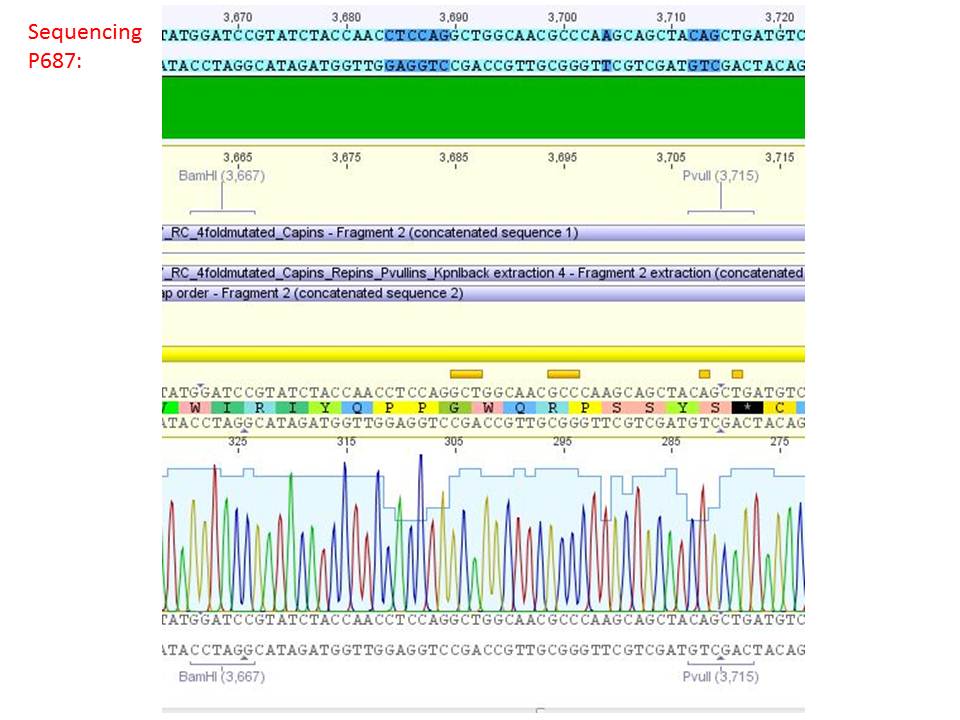
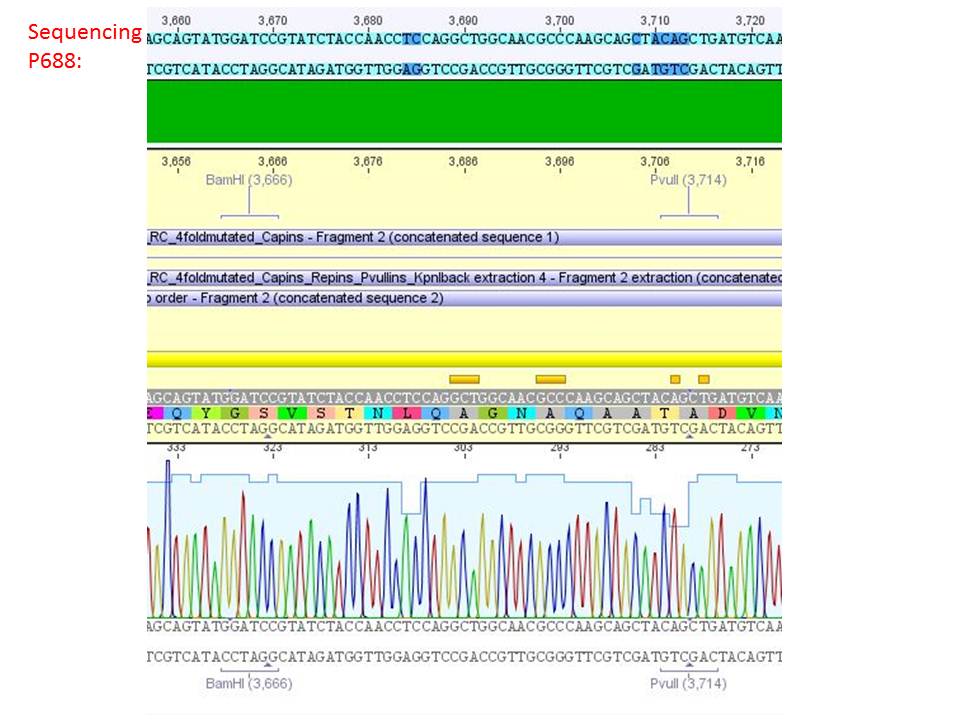
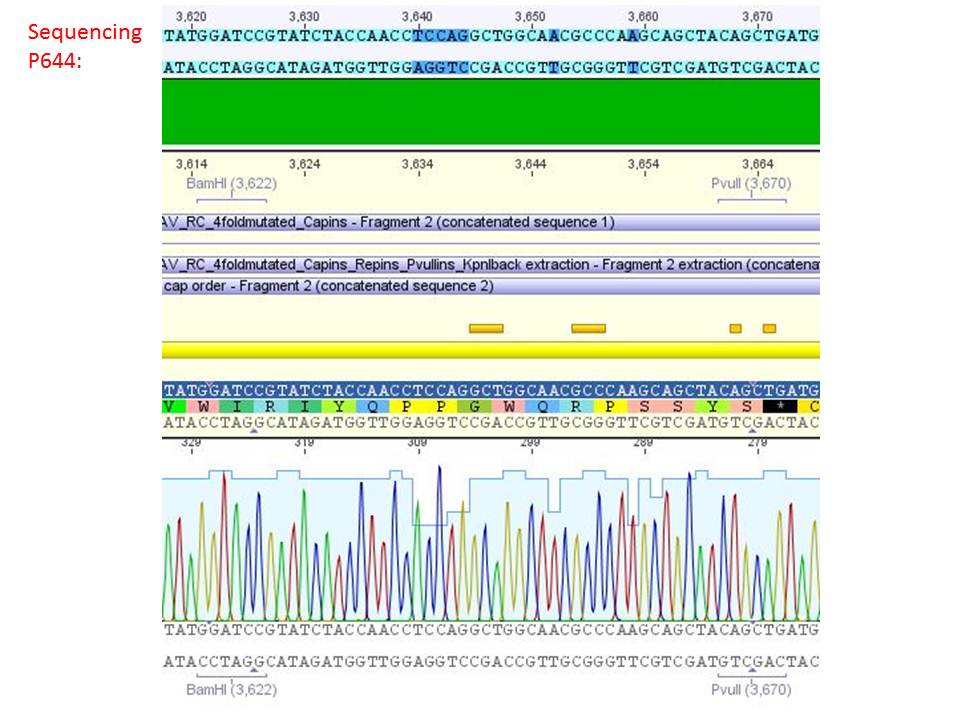
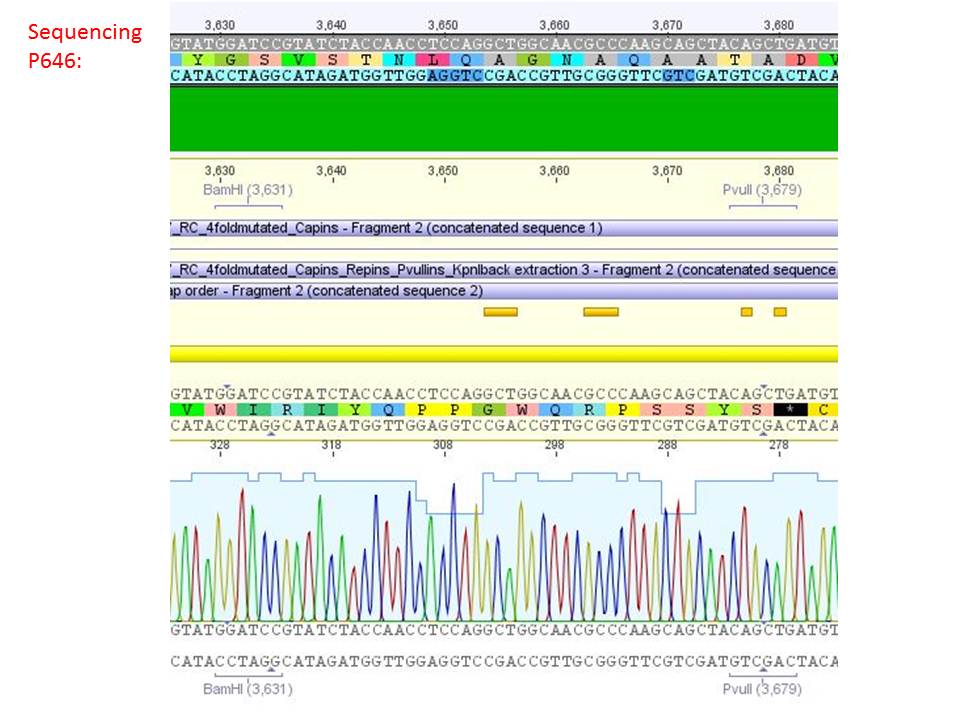
Sequencing analysis of HSPG-ko in several IRCK constructs
Investigator: Stefan
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 1 (P687):
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 2(P688):
pSB1C3_001_RC_IRCK_VP1-ko_HSPG-ko_P5tataless cl1 (P644):
pSB1C3_001_RC_IRCK_VP2-ko_HSPG-ko_P5tataless cl1 (P646):
Comment: Sequencing results show that all plasmids contain the HSGP-ko. Working will be continued using P644, P646 and P687.
Mini Prep of pGa14_MiddleLinker_VP2/3_inscap and pSB1C3_Darpin
Investigator: Jessica
- pSB1C3_Darpin clone1 c=158.5 ng/µl
- pSB1C3_Darpin clone2 c=205.6 ng/µl
- pGA14_MiddleLinker_VP2/3_inscap clone 1 c=147.5 ng/µl
- pGA14_MiddleLinker_VP2/3_inscap clone 2 c=121.0 ng/µl
Test-digestion:
| Components | pSB1C3_Darpin clone1/2 |
| DNA | 1,5 µl |
| BSA (10x) | 1 µl |
| Buffer no. 4 | 1 µl |
| Enzyme 1 XbaI | 0,4 µl |
| Enzyme 2 AgeI | 0,4 µl |
| H2O | 5,7 µl |
| Total volume | 10 |
Hanna wrote that a 3rd enzyme is need for the test digestion of pGA14_MiddleLinker_VP2/3_inscap but I couln't find any sequence of pGA14_MiddleLinker_VP2/3_inscap or pGA14

Mass Midi-Prep II
Investigator: Chris W.
Midi-Prep of:
453_BAP =P631
587_BAP =P632
587_KO_BAP =P633
453_RGD =P634
587_RGD =P635
587_KO_RGD =P636
453_HIS =P637
587_HIS =P638
587_KO_HIS =P639
587_KO_empty =P640
453_Z34C =P641
pSB1C3_001_RC_IRCK_VP1-ko_P5tataless =P642 =B463
pSB1C3_001_RC_IRCK_VP2-ko_P5tataless =P643 =B470
The Midi-Preps were performed according to the standard protocol yielding the following concentrations:
| plasmid-no. | P631 | P632 | P633 | P634 | P635 | P636 | P637 | P638 | P639 | P640 | P641 | P642 | P643 |
| concentration (ng/µl) | 4758,69 | 448,12 | 3243,97 | 2231,44 | 1401,68 | 3289,15 | 3441,22 | 3058,92 | 4425,05 | 2339,06 | 4377,05 | 5045,55 | 5201,73 |
 "
"