Team:HokkaidoU Japan/Protocols
From 2010.igem.org
(Difference between revisions)
(Removing all content from page) |
|||
| Line 1: | Line 1: | ||
| + | {{Template:HokkaidoU_Japan}} | ||
| + | =Protocols= | ||
| + | <ul class="acc" id="acc"> | ||
| + | <li> | ||
| + | ==Preparation of Competent cells (''E. coli'' DH5a)== | ||
| + | <div class="acc-section"> | ||
| + | <div class="acc-content"> | ||
| + | ===Reagents=== | ||
| + | '''TB (Transformation Buffer)(at 4C, filtration)''' | ||
| + | {|border="1" class="protocol" | ||
| + | | | ||
| + | | | ||
| + | |Final concentration | ||
| + | |- | ||
| + | |1 M CaCl<sub>2</sub> (at RT, autoclaved) | ||
| + | |0.75 mL | ||
| + | |15 mM | ||
| + | |- | ||
| + | |4 M KCl (at RT, autoclaved) | ||
| + | |3.125 mL | ||
| + | |250 mM | ||
| + | |- | ||
| + | |1 M MnCl<sub>2</sub> (at 4C, autoclaved) | ||
| + | |2.75 mL | ||
| + | |55 mM | ||
| + | |- | ||
| + | |1 M PIPES (pH 6.7 by NaOH, at 4C, filtration) | ||
| + | |0.5 mL | ||
| + | |10 mM | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''50 mL''' | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | ===Procedure=== | ||
| + | # Single colony isolation on LB plate | ||
| + | # incubate the plate for 15-19 hrs at 37C | ||
| + | # lift a colony into 2 mL of LB | ||
| + | # culture cells at 37C for 12-16 hrs at 180-200 rpm | ||
| + | # transfer 30 uL, 100 uL, 300 uL of the culture into 100 mL SOB medium, respectively | ||
| + | # culture cells at 20C (for 24 hrs over) at 180-200 rpm (to ΔOD<sub>550nm</sub> = 0.5~0.6) | ||
| + | # leave the 300 mL flask for 10 min on ice | ||
| + | # transfer the culture into two 50 mL Falcon tube | ||
| + | # centrifuge 7500 rpm at 4C for 20 min (TOMY TA-22 rotor), and discard sup | ||
| + | # suspend the pellet in ice-cold 15 mL of TB (Transformation Buffer)(7.5 mL/tube) | ||
| + | # centrifuge 7500 rpm at 4C for 2 min (TOMY TA-22 rotor), and discard sup | ||
| + | # suspend the pellet in ice-cold 3.2 mL of TB | ||
| + | # add 0.24 mL of DMSO (stirring, bit by bit) | ||
| + | # leave the 50 mL Falcon tube for 10 min on ice | ||
| + | # dispense 50 uL into 0.5 mL tube | ||
| + | # freeze the suspension in liquid nitrogen | ||
| + | # store at -80C | ||
| + | </div></div> | ||
| + | </li> | ||
| + | <li> | ||
| + | ==Bacterial Transformations== | ||
| + | <div class="acc-section"> | ||
| + | <div class="acc-content"> | ||
| + | # add DNA solution to thawed competent cells | ||
| + | # incubate the cells on ice for 30 min | ||
| + | # heat shock the cells by immersion in a pre-heated water bath at 42C for 60 sec | ||
| + | # incubate the cells on ice for 5 min | ||
| + | # add 200 uL of SOB broth | ||
| + | # incubate the cells at 37C for 2 hrs while the tubes are shaking | ||
| + | # plate 200 uL of the transformation onto the dish | ||
| + | # incubate the plate at 37C for 12-14 hrs | ||
| + | </div></div> | ||
| + | </li> | ||
| + | <li> | ||
| + | |||
| + | ==Mini-prep (Alkaline SDS Method)== | ||
| + | <div class="acc-section"><div class="acc-content"> | ||
| + | ===Reagents=== | ||
| + | '''Solution I''' | ||
| + | (at RT, filtration 0.2 um, 50 mL) | ||
| + | {|border="1" class="protocol" | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | |Final concentration | ||
| + | |- | ||
| + | |Glucose (at RT) | ||
| + | |0.45 g | ||
| + | |50 mM | ||
| + | |- | ||
| + | |1 M Tris-HCl (pH8.0, at RT, autoclaved) | ||
| + | |1.25 mL | ||
| + | |25 mM | ||
| + | |- | ||
| + | |0.5 M EDTA (pH8.0, at RT, autoclaved) | ||
| + | |1 mL | ||
| + | |10 mM | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''50 mL''' | ||
| + | | | ||
| + | |} | ||
| + | <br> | ||
| + | |||
| + | '''Solution II''' | ||
| + | (at RT, filtration 0.2 um, 20 mL) | ||
| + | {|border="1" class="protocol" | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | |Final concentration | ||
| + | |- | ||
| + | |10 N NaOH (at RT) | ||
| + | |0.4 mL | ||
| + | |0.2 N | ||
| + | |- | ||
| + | |10% SDS (at RT, filtration) | ||
| + | |2 mL | ||
| + | |1% | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''20 mL''' | ||
| + | | | ||
| + | |} | ||
| + | <br> | ||
| + | |||
| + | '''Solution III''' | ||
| + | (at RT, filtration 0.2 um, 50 mL) | ||
| + | {|border="1" class="protocol" | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | |Final concentration | ||
| + | |- | ||
| + | |5 M CH<sub>3</sub>COOK | ||
| + | |30 mL | ||
| + | |3 M | ||
| + | |- | ||
| + | |CH<sub>3</sub>COOH | ||
| + | |5.75 mL | ||
| + | | | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |14.25 mL | ||
| + | | | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''50 mL''' | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | ===Procedure=== | ||
| + | # lift colony ''E. coli'' into 2 mL LB contained antibiotics | ||
| + | # culture cells at 37C for 16-20 hrs at 180-200 rpm | ||
| + | # transfer 1.2-1.5 mL of culture into 1.5 mL tube | ||
| + | # centrifuge the culture at 15,000 rpm for 1 min at 4C and discard sup | ||
| + | # suspend the pellet in ice-cold 100 uL of Solution I | ||
| + | # add 200 uL of Solution II to the suspension | ||
| + | # mix by inverting the tube 10-20 times | ||
| + | # add ice-cold 150 uL of Solution III to the suspension | ||
| + | # mix by inverting the tube 10-20 times | ||
| + | # leave the tube for 5 min on ice | ||
| + | # add 10 uL of Chloroform | ||
| + | # mix by inverting the tube 5-10 times | ||
| + | # centrifuge the suspension at 15,000 rpm for 5 min at 4C | ||
| + | # transfer the supernatant into new 1.5 mL tube↓ | ||
| + | # add equal volume of isopropanol and mix by voltexing | ||
| + | # leave the tube for 5 min at RT | ||
| + | # centrifuge the suspension at 15,000 rpm for 10 min at 4C and discard sup | ||
| + | # rinse the ppt by 70% EtOH and mix by voltexing | ||
| + | # centrifuge the suspension at 15,000 rpm for 2 min at 4C and discard sup | ||
| + | # dry up the ppt | ||
| + | # dissolve the ppt in 50 uL of TE (pH 8.0) | ||
| + | # add 1 uL of 10 mg/mL RNase A (4C and stock at –20C) | ||
| + | # incubate for 30 min at 37C | ||
| + | # PCIAA and CIAA extraction | ||
| + | # Ethanol precipitation | ||
| + | # dry up the ppt | ||
| + | # dissolve the ppt in 50 uL of TE (pH 8.0) | ||
| + | |||
| + | </div></div></li><li> | ||
| + | |||
| + | ==PCR== | ||
| + | <div class="acc-section"><div class="acc-content"> | ||
| + | ===Vector=== | ||
| + | '''Standard reaction setup''' | ||
| + | {|class="protocol" style="text-align:center;" border="1" | ||
| + | |- | ||
| + | !Component | ||
| + | !Volume | ||
| + | |- | ||
| + | |10x PCR Buffer | ||
| + | |5 uL | ||
| + | |- | ||
| + | |2mM dNTPs | ||
| + | |5 uL | ||
| + | |- | ||
| + | |25mM MgSO<sub>4</sub> | ||
| + | |3 uL | ||
| + | |- | ||
| + | |Suffix-F primer | ||
| + | |1 uL | ||
| + | |- | ||
| + | |Prefix-R primer | ||
| + | |1 uL | ||
| + | |- | ||
| + | |Template DNA | ||
| + | |1 uL | ||
| + | |- | ||
| + | |KOD -Plus- Neo | ||
| + | |1 uL | ||
| + | |- | ||
| + | |DW | ||
| + | |X uL | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''50 uL''' | ||
| + | |} | ||
| + | |||
| + | '''Cycling conditions ''' (2-step cycle) | ||
| + | {|class="protocol" border="1" | ||
| + | |- | ||
| + | | Predenature | ||
| + | | 94C 2 min | ||
| + | |- | ||
| + | | Denature | ||
| + | | 98C 10 sec | ||
| + | |- | ||
| + | | Extension | ||
| + | | 68C X sec (30 sec/kb) | ||
| + | |- | ||
| + | | Hold | ||
| + | | 4C | ||
| + | |} | ||
| + | * 30-40 cycles | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ===Insert=== | ||
| + | '''Standard reaction setup''' | ||
| + | {|class="protocol" style="text-align:center;" border="1" | ||
| + | |- | ||
| + | !Component | ||
| + | !Volume | ||
| + | |- | ||
| + | |10x PCR Buffer | ||
| + | |5 uL | ||
| + | |- | ||
| + | |2mM dNTPs | ||
| + | |5 uL | ||
| + | |- | ||
| + | |25mM MgSO<sub>4</sub> | ||
| + | |3 uL | ||
| + | |- | ||
| + | |EX-F primer | ||
| + | |1 uL | ||
| + | |- | ||
| + | |PS-R primer | ||
| + | |1 uL | ||
| + | |- | ||
| + | |Template DNA | ||
| + | |1 uL | ||
| + | |- | ||
| + | |KOD -Plus- Neo | ||
| + | |1 uL | ||
| + | |- | ||
| + | |DW | ||
| + | |X uL | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''50 uL''' | ||
| + | |} | ||
| + | |||
| + | '''Cycling conditions ''' (2-step cycle) | ||
| + | {|class="protocol" border="1" | ||
| + | |- | ||
| + | | Predenature | ||
| + | | 94C 2 min | ||
| + | |- | ||
| + | | Denature | ||
| + | | 98C 10 sec | ||
| + | |- | ||
| + | | Extension | ||
| + | | 68C X sec (30 sec/kb) | ||
| + | |- | ||
| + | | Hold | ||
| + | | 4C | ||
| + | |} | ||
| + | * 30-40 cycles | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | * resuspend a colony into 10 uL of DW (template suspension) | ||
| + | * | ||
| + | '''Standard reaction setup''' | ||
| + | {|class="protocol" style="text-align:center;" border="1" | ||
| + | |- | ||
| + | !Component | ||
| + | !Volume | ||
| + | |- | ||
| + | |template suspension | ||
| + | |4.8 uL | ||
| + | |- | ||
| + | |Quick Taq | ||
| + | |5 uL | ||
| + | |- | ||
| + | |Forward primer | ||
| + | |0.1 uL | ||
| + | |- | ||
| + | |Reverse primer | ||
| + | |0.1 uL | ||
| + | |- | ||
| + | |'''Total''' | ||
| + | |'''10 uL''' | ||
| + | |} | ||
| + | |||
| + | '''Cycling conditions ''' (2-step cycle) | ||
| + | {|class="protocol" border="1" | ||
| + | |- | ||
| + | | Predenature | ||
| + | | 94C 2 min | ||
| + | |- | ||
| + | | Denature | ||
| + | | 94C 10 sec | ||
| + | |- | ||
| + | | Extension | ||
| + | | 68C X sec (60 sec/kb) | ||
| + | |- | ||
| + | | Hold | ||
| + | | 4C | ||
| + | |} | ||
| + | * 30-40 cycles | ||
| + | |||
| + | </div></div></li><li> | ||
| + | |||
| + | ==Restriction Enzyme Digestions== | ||
| + | <div class="acc-section"><div class="acc-content">c</div></div></li><li> | ||
| + | ==DNA ligation== | ||
| + | <div class="acc-section"><div class="acc-content">d</div></div></li><li> | ||
| + | ==Agarose gel electrophoresis== | ||
| + | <div class="acc-section"><div class="acc-content"> | ||
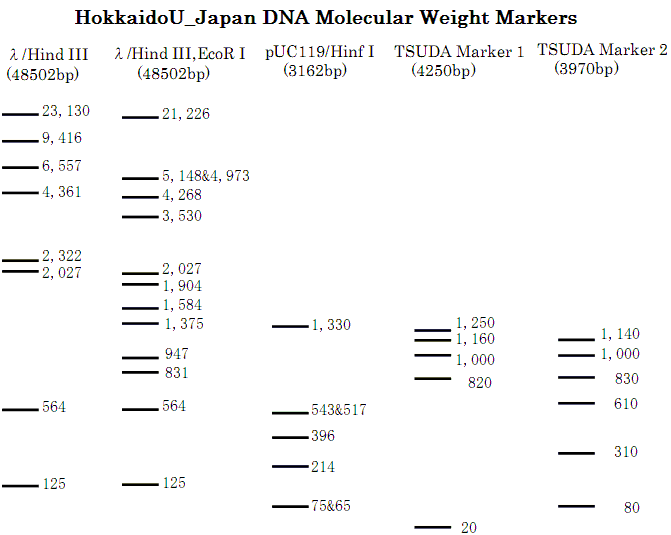
| + | [[Image:HokkaidoU_Pictures_DNA_Marker.png|200px|thumb|right|DNA Weight Markers]]</div></div></li><li> | ||
| + | |||
| + | ==Electroporation== | ||
| + | <div class="acc-section"><div class="acc-content"> | ||
| + | '''Preparation of electro-competent cells''' | ||
| + | # cell culture in 400 mL of SOB or LB and grow to ΔOD<sub>600</sub> = 0.5~0.6 | ||
| + | # dispense the medium into 8 Falcon 50 mL tube | ||
| + | # centrifuge at 3500 rpm for 5 min at 4C and discard sup | ||
| + | # add 5 mL of DW and suspend the ppt, mix 8 suspensions into single Falcon tube | ||
| + | # centrifuge at 3500 rpm for 5 min at 4C and discard sup | ||
| + | # add 40 mL of DW and suspend the ppt | ||
| + | # centrifuge at 3500 rpm for 5 min at 4C and discard sup | ||
| + | # add 10 mL of 10% Glycerol and suspend the ppt | ||
| + | # centrifuge at 3500 rpm for 5 min at 4C and discard sup | ||
| + | # add 10 mL of 10% Glycerol and suspend the ppt | ||
| + | # centrifuge at 3500 rpm for 5 min at 4C and discard sup | ||
| + | # add 5 mL of 10% Glycerol and suspend the ppt | ||
| + | # dispense 100 uL of the suspensions into 0.5 mL Eppendorf tube, respectively | ||
| + | # store at -80C freezer | ||
| + | <br> | ||
| + | '''Electroporation''' | ||
| + | |||
| + | |||
| + | |||
| + | </div></div></li><li> | ||
| + | ==PCIAA and CIAA extraction== | ||
| + | <div class="acc-section"><div class="acc-content"> | ||
| + | '''Reagent''' | ||
| + | * PCIAA = Phenol : Chloroform : IsoAmyl Alcohol = 25 : 24 : 1 | ||
| + | * CIAA = Chloroform : IsoAmyl Alcohol = 24 : 1 | ||
| + | |||
| + | '''Procedure''' | ||
| + | # add equal volume of PCIAA and vortex vigorously | ||
| + | # centrifuge at 15,000 rpm for 2 min at RT | ||
| + | # transfer the aqueous phase to a new tube, being careful not to transfer the phase interface | ||
| + | # add equal volume of CIAA and vortex vigorously | ||
| + | # transfer the aqueous phase to a new tube | ||
| + | # ethanol precipitation | ||
| + | |||
| + | |||
| + | </div></div></li><li> | ||
| + | ==Ethanol presipitation== | ||
| + | <div class="acc-section"><div class="acc-content"> | ||
| + | # add 1/10 volume of 3M CH<sub>3</sub>COONa | ||
| + | # add 2.5 volume of 100% ethanol (EtOH) | ||
| + | # incubate on ice for few min | ||
| + | # centrifuge at 15,000 rpm for 10 min at 4C and discard sup | ||
| + | # wash precipitation with 100 uL of 70% EtOH (EtOH has to be cold) | ||
| + | # centrifuge at 15,000 rpm for 5 min at 4C and discard sup | ||
| + | # dry up the ppt (no EtOH should be left) | ||
| + | # resuspend ppt in wanted volume of TE | ||
| + | |||
| + | |||
| + | </div></div></li></ul> | ||
| + | |||
| + | <html> | ||
| + | <script type="text/javascript" src="http://igemsapporo.com/scripts/accordion.js"></script> | ||
| + | <script type="text/javascript"> | ||
| + | var acc=new TINY.accordion.slider("acc"); | ||
| + | acc.init("acc","h2",0,-1); | ||
| + | </script> | ||
| + | </html> | ||
Revision as of 10:18, 25 September 2010
Protocols
-
Preparation of Competent cells (E. coli DH5a)
Reagents
TB (Transformation Buffer)(at 4C, filtration)
Final concentration 1 M CaCl2 (at RT, autoclaved) 0.75 mL 15 mM 4 M KCl (at RT, autoclaved) 3.125 mL 250 mM 1 M MnCl2 (at 4C, autoclaved) 2.75 mL 55 mM 1 M PIPES (pH 6.7 by NaOH, at 4C, filtration) 0.5 mL 10 mM Total 50 mL Procedure
- Single colony isolation on LB plate
- incubate the plate for 15-19 hrs at 37C
- lift a colony into 2 mL of LB
- culture cells at 37C for 12-16 hrs at 180-200 rpm
- transfer 30 uL, 100 uL, 300 uL of the culture into 100 mL SOB medium, respectively
- culture cells at 20C (for 24 hrs over) at 180-200 rpm (to ΔOD550nm = 0.5~0.6)
- leave the 300 mL flask for 10 min on ice
- transfer the culture into two 50 mL Falcon tube
- centrifuge 7500 rpm at 4C for 20 min (TOMY TA-22 rotor), and discard sup
- suspend the pellet in ice-cold 15 mL of TB (Transformation Buffer)(7.5 mL/tube)
- centrifuge 7500 rpm at 4C for 2 min (TOMY TA-22 rotor), and discard sup
- suspend the pellet in ice-cold 3.2 mL of TB
- add 0.24 mL of DMSO (stirring, bit by bit)
- leave the 50 mL Falcon tube for 10 min on ice
- dispense 50 uL into 0.5 mL tube
- freeze the suspension in liquid nitrogen
- store at -80C
-
Bacterial Transformations
- add DNA solution to thawed competent cells
- incubate the cells on ice for 30 min
- heat shock the cells by immersion in a pre-heated water bath at 42C for 60 sec
- incubate the cells on ice for 5 min
- add 200 uL of SOB broth
- incubate the cells at 37C for 2 hrs while the tubes are shaking
- plate 200 uL of the transformation onto the dish
- incubate the plate at 37C for 12-14 hrs
-
Mini-prep (Alkaline SDS Method)
Reagents
Solution I (at RT, filtration 0.2 um, 50 mL)
Final concentration Glucose (at RT) 0.45 g 50 mM 1 M Tris-HCl (pH8.0, at RT, autoclaved) 1.25 mL 25 mM 0.5 M EDTA (pH8.0, at RT, autoclaved) 1 mL 10 mM Total 50 mL
Solution II (at RT, filtration 0.2 um, 20 mL)
Final concentration 10 N NaOH (at RT) 0.4 mL 0.2 N 10% SDS (at RT, filtration) 2 mL 1% Total 20 mL
Solution III (at RT, filtration 0.2 um, 50 mL)
Final concentration 5 M CH3COOK 30 mL 3 M CH3COOH 5.75 mL H2O 14.25 mL Total 50 mL Procedure
- lift colony E. coli into 2 mL LB contained antibiotics
- culture cells at 37C for 16-20 hrs at 180-200 rpm
- transfer 1.2-1.5 mL of culture into 1.5 mL tube
- centrifuge the culture at 15,000 rpm for 1 min at 4C and discard sup
- suspend the pellet in ice-cold 100 uL of Solution I
- add 200 uL of Solution II to the suspension
- mix by inverting the tube 10-20 times
- add ice-cold 150 uL of Solution III to the suspension
- mix by inverting the tube 10-20 times
- leave the tube for 5 min on ice
- add 10 uL of Chloroform
- mix by inverting the tube 5-10 times
- centrifuge the suspension at 15,000 rpm for 5 min at 4C
- transfer the supernatant into new 1.5 mL tube↓
- add equal volume of isopropanol and mix by voltexing
- leave the tube for 5 min at RT
- centrifuge the suspension at 15,000 rpm for 10 min at 4C and discard sup
- rinse the ppt by 70% EtOH and mix by voltexing
- centrifuge the suspension at 15,000 rpm for 2 min at 4C and discard sup
- dry up the ppt
- dissolve the ppt in 50 uL of TE (pH 8.0)
- add 1 uL of 10 mg/mL RNase A (4C and stock at –20C)
- incubate for 30 min at 37C
- PCIAA and CIAA extraction
- Ethanol precipitation
- dry up the ppt
- dissolve the ppt in 50 uL of TE (pH 8.0)
-
PCR
Vector
Standard reaction setup
Component Volume 10x PCR Buffer 5 uL 2mM dNTPs 5 uL 25mM MgSO4 3 uL Suffix-F primer 1 uL Prefix-R primer 1 uL Template DNA 1 uL KOD -Plus- Neo 1 uL DW X uL Total 50 uL Cycling conditions (2-step cycle)
Predenature 94C 2 min Denature 98C 10 sec Extension 68C X sec (30 sec/kb) Hold 4C - 30-40 cycles
Insert
Standard reaction setup
Component Volume 10x PCR Buffer 5 uL 2mM dNTPs 5 uL 25mM MgSO4 3 uL EX-F primer 1 uL PS-R primer 1 uL Template DNA 1 uL KOD -Plus- Neo 1 uL DW X uL Total 50 uL Cycling conditions (2-step cycle)
Predenature 94C 2 min Denature 98C 10 sec Extension 68C X sec (30 sec/kb) Hold 4C - 30-40 cycles
Colony PCR
- resuspend a colony into 10 uL of DW (template suspension)
Standard reaction setup
Component Volume template suspension 4.8 uL Quick Taq 5 uL Forward primer 0.1 uL Reverse primer 0.1 uL Total 10 uL Cycling conditions (2-step cycle)
Predenature 94C 2 min Denature 94C 10 sec Extension 68C X sec (60 sec/kb) Hold 4C - 30-40 cycles
-
Restriction Enzyme Digestions
c -
DNA ligation
d -
Agarose gel electrophoresis
-
Electroporation
Preparation of electro-competent cells
- cell culture in 400 mL of SOB or LB and grow to ΔOD600 = 0.5~0.6
- dispense the medium into 8 Falcon 50 mL tube
- centrifuge at 3500 rpm for 5 min at 4C and discard sup
- add 5 mL of DW and suspend the ppt, mix 8 suspensions into single Falcon tube
- centrifuge at 3500 rpm for 5 min at 4C and discard sup
- add 40 mL of DW and suspend the ppt
- centrifuge at 3500 rpm for 5 min at 4C and discard sup
- add 10 mL of 10% Glycerol and suspend the ppt
- centrifuge at 3500 rpm for 5 min at 4C and discard sup
- add 10 mL of 10% Glycerol and suspend the ppt
- centrifuge at 3500 rpm for 5 min at 4C and discard sup
- add 5 mL of 10% Glycerol and suspend the ppt
- dispense 100 uL of the suspensions into 0.5 mL Eppendorf tube, respectively
- store at -80C freezer
Electroporation
-
PCIAA and CIAA extraction
Reagent
- PCIAA = Phenol : Chloroform : IsoAmyl Alcohol = 25 : 24 : 1
- CIAA = Chloroform : IsoAmyl Alcohol = 24 : 1
Procedure
- add equal volume of PCIAA and vortex vigorously
- centrifuge at 15,000 rpm for 2 min at RT
- transfer the aqueous phase to a new tube, being careful not to transfer the phase interface
- add equal volume of CIAA and vortex vigorously
- transfer the aqueous phase to a new tube
- ethanol precipitation
-
Ethanol presipitation
- add 1/10 volume of 3M CH3COONa
- add 2.5 volume of 100% ethanol (EtOH)
- incubate on ice for few min
- centrifuge at 15,000 rpm for 10 min at 4C and discard sup
- wash precipitation with 100 uL of 70% EtOH (EtOH has to be cold)
- centrifuge at 15,000 rpm for 5 min at 4C and discard sup
- dry up the ppt (no EtOH should be left)
- resuspend ppt in wanted volume of TE
 "
"