Team:Stanford/Notebook/Lab Work/Week 2
From 2010.igem.org
(→Laura's Lab Notebook) |
(→7/9 Friday) |
||
| (9 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
==7/5 Monday== | ==7/5 Monday== | ||
| + | |||
| + | University Holiday: Planning Meeting moved to tomorrow | ||
| + | |||
| + | ==7/6 Tuesday== | ||
| + | |||
===Planning Meeting Notes=== | ===Planning Meeting Notes=== | ||
| Line 43: | Line 48: | ||
**design standard sRNA designs and place order by the end of the week | **design standard sRNA designs and place order by the end of the week | ||
| - | == | + | === 2:00 Meeting with Christina (Karina, Francisco, Laura)=== |
| + | to test: sRNA complementary to ID-RBS-scar; another sRNA centered across RBS as control (to see if shifting will affect efficiency of sRNA) | ||
| + | |||
| + | To Do: | ||
| + | #check registry for shorter pBAD promoters? PCR own modified version? | ||
| + | #finalize ID's | ||
| + | *RNA folding: avoid local strong structures, > -10kcal/mol good: look at free energies of individual hairpins | ||
| + | *BLAST to make sure not in E. Coli genome | ||
| + | |||
===Alex's Notebook=== | ===Alex's Notebook=== | ||
| Line 75: | Line 88: | ||
| plasmid backbone || pSB1K3 || P1 5A || Kan | | plasmid backbone || pSB1K3 || P1 5A || Kan | ||
|} | |} | ||
| + | ===Chris's Notebook=== | ||
| + | 7/6/2010 | ||
| + | Goals for Today: Restriction Digestion of Promoters (pBAD and F2620), GFP reporter (E0240), and plasmid. Ligation of promoters, reporters, and plasmids. | ||
| + | Chris: | ||
| + | 4A5 E/P 3395 bp | ||
| + | I0500 E/S (on psb2K3) 1210 bp | ||
| + | F2620 E/S (on pSB1A2) 1061 bp | ||
| + | Ryan’s Protocol for Restriction Digest | ||
| + | Cloning Digestion (50μl reaction volume) | ||
| + | |||
| + | *Plasmid 12.0 μl | ||
| + | *Each enzyme 1.0 μl | ||
| + | *NEB buffer 5.0 μl | ||
| + | *10x BSA 0 or 5 μl (optional depending on enzymes used) | ||
| + | *NP water Bring to 50 μl total volume (26 ul) | ||
| + | *Let sit for at least an hour at 37 degrees C | ||
| + | Heat Kill for 20 minutes at 65 degrees C | ||
| + | |||
| + | *Note: Jerome’s I0500 is extremely concentrated (1.25 ug/ul). | ||
| + | *Restriction Digest ran overnight until 940 am the next morning | ||
==7/7 Wednesday== | ==7/7 Wednesday== | ||
| Line 93: | Line 126: | ||
*Transformed E.coli with E1010 (RFP) using heatshock. Used 1uL DNA from distribution. | *Transformed E.coli with E1010 (RFP) using heatshock. Used 1uL DNA from distribution. | ||
*Transferred 50uL of transformed cells to agar plate with kanamycin. | *Transferred 50uL of transformed cells to agar plate with kanamycin. | ||
| + | |||
| + | ===Chris's Notebook=== | ||
| + | |||
| + | Running a Gel of F2620, pBAD, and psb4A5: | ||
| + | *Make a 0.8% agarose gel (0.4 g of agarose in 50 ml of TAE) | ||
| + | -Heat in microwave until agarose is completely dissolved | ||
| + | -Add 10.0 ul of EtBr to mix (5000x for a 50 ml solution) | ||
| + | *let run for approximately 1 hr | ||
| + | *refer below for bands: | ||
| + | 4A5 E/P 3395 bp | ||
| + | I0500 (pBAD) E/S (on psb2K3) 1210 bp | ||
| + | F2620 E/S (on pSB1A2) 1061 bp | ||
| + | |||
| + | ====Making Kanamycin, Ampicillin Chloramphenicol Plates==== | ||
| + | Kanamycin is at 1000x | ||
| + | |||
| + | ====PCR of I746908 to isolate the RBS, CDS, and double terminators==== | ||
| + | Programming the Block: | ||
| + | *1. 95 C for 2 minutes | ||
| + | *2. 95 C for 30 sec | ||
| + | *3. 55 C for 30 sec | ||
| + | *4. 68 C for 1.10 min | ||
| + | *5. go to step 2 for 30 cycles | ||
| + | *6. 72 for 5 minutes | ||
| + | *7. end (0=forever) | ||
| + | Use PCR HF SuperMix | ||
| + | For each reaction: | ||
| + | 1.25 ul of forward primer | ||
| + | 1.25 ul of reverse primer | ||
| + | 1.0 ul of DNA template | ||
| + | 46.5 ul of PCR HF SuperMix | ||
==7/8 Thursday== | ==7/8 Thursday== | ||
| Line 271: | Line 335: | ||
*worked with Karina and Francisco on digestions, gel, gel extraction (see Karina's notebook for details) | *worked with Karina and Francisco on digestions, gel, gel extraction (see Karina's notebook for details) | ||
*for digestion: Francisco set up RFP, Karina set up terminator, I set up GFP | *for digestion: Francisco set up RFP, Karina set up terminator, I set up GFP | ||
| + | |||
| + | ===Chris's Notebook=== | ||
| + | ====Completion of PCR==== | ||
| + | Note: only use PCR cleanup if the DNA is going to be used for ligation | ||
| + | ====Running a Gel of PCR Product:==== | ||
| + | * first need to PCR cleanup | ||
| + | *then run on 1.4% agarose gel (0.7 g of agarose in 50 ml of TAE) | ||
| + | *this is necessary because of the 800 bp size of the fragment | ||
| + | *run at 85 volts | ||
| + | *small combs | ||
| + | |||
| + | Restriction Digest of I746908-E/P | ||
| + | *Cloning Digestion (50μl reaction volume): | ||
| + | 4A5 E/P 3395 bp | ||
| + | I0500 E/S (on psb2K3) 1210 bp | ||
| + | F2620 E/S (on pSB1A2) 1061 bp | ||
| + | 3C5 E/P 2738 bp (buffer 3) | ||
| + | F2620 E/S (on pSB1A2) 1061 bp | ||
| + | |||
| + | ====Run Gels of Restriction Digests==== | ||
| + | *run on 1.4% agarose gel (0.7 g of agarose in 50 ml of TAE) | ||
| + | *this is necessary because of the 800 bp size of the fragment | ||
| + | *run at 85 volts | ||
| + | *small combs | ||
| + | Expected Sizes: | ||
| + | 3C5 E/P 2738 bp (buffer 3) | ||
| + | F2620 E/S (on pSB1A2) 1061 bp | ||
| + | I746908 E/P (on psb1A2) 2093 bp | ||
| + | B0034 X/P (on psb1A2) 12 bp-do NOT run on gel | ||
| + | J23100 E/S (on psb1A2) 35 bp-do NOT run on gel | ||
| + | |||
| + | Gel Extract | ||
| + | |||
| + | ==7/10 Saturday== | ||
| + | |||
| + | Run gels for the restriction digests. 2 failed due to similar sizes | ||
| + | Could not ligate current pieces. | ||
| + | |||
| + | New Reporter | ||
| + | *I746916 (N/A) | ||
| + | |||
| + | |||
| + | ==7/11 Sunday== | ||
| + | |||
| + | Prepared new triple restriction digests with EcoRI, PstI, and BglI. | ||
| + | *I8 and T9. | ||
| + | |||
| + | Inoculated 3C5, 1K3, I0500, and I8 to obtain more DNA. | ||
| + | |||
</div> | </div> | ||
==7/9 Friday== | ==7/9 Friday== | ||
Latest revision as of 02:05, 14 September 2010

| Home | Project | Applications | Modeling | Parts | Team | Notebook |
Spring: Brainstorming | Spring Meetings
Summer: Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Summaries
|
7/5 Monday
University Holiday: Planning Meeting moved to tomorrow
7/6 Tuesday
Planning Meeting Notes
Agenda
- Design Updates
- Analog Comparator (Chris & Alex)
- Redundancy between the two proposals
- Degree of phosporylation
- Dynamic range and mechanisms
- Digital Comparator (Francisco & Karina)
- RSID; standardizing the design
- Introducing a PoPs signal output
- Characteristics between the two Comparators
- What makes the two different?
- Applications
- Vaginal Infections/Preterm Birth
- is it really a ratio?
- Other ideas: Targeting Cancer Cells, oscillators
- Administrative Details
- Ordering Stuff: Oligos ordering sheet, Digestion and Gel Extraction Kits?
- Stipend?
- Sponsorship Updates
- MDV Presentation
Goals For the Week
- Chris & Alex
- need to make 50x TAE
- complete plasmids as far can get w/o kinase and phosphotase
- order DNA sometime this week
- Francisco & Karina
- Run orginal and new designs by Christina
- design standard sRNA designs and place order by the end of the week
2:00 Meeting with Christina (Karina, Francisco, Laura)
to test: sRNA complementary to ID-RBS-scar; another sRNA centered across RBS as control (to see if shifting will affect efficiency of sRNA)
To Do:
- check registry for shorter pBAD promoters? PCR own modified version?
- finalize ID's
- RNA folding: avoid local strong structures, > -10kcal/mol good: look at free energies of individual hairpins
- BLAST to make sure not in E. Coli genome
Alex's Notebook
- Restriction Digests:
- Take the part out completely, and/or take out either prefix or suffix.
- KEEP SOME DNA FOR GEL CONTROL!!!
- 2K3 E/P
- 1A2 E/P
- E0240 1A2; X/P length: 876
- T9002 1A3; E/P length: 1945
Greg's Notebook
- During weekly meeting got up to speed with what the team’s been doing while I was away
- Used heat-shock transformation protocol for DH5alpha to transform with the following genetic material, left to culture overnight:
| Function | Part # | Distribution Location | Resistance |
|---|---|---|---|
| ribosome binding site | B0032 | P1 2I | Amp |
| forward double terminator | B0015 | P1 23L | Amp + Kan |
| bidirectional double terminator | B0014 | P2 24C | Amp + Kan |
| medium constitutive promoter | J23107 | P1 20A | Amp |
| strong constitutive promoter | J23100 | P1 18C | Amp |
| plasmid backbone | pSB1K3 | P1 5A | Kan |
Chris's Notebook
7/6/2010 Goals for Today: Restriction Digestion of Promoters (pBAD and F2620), GFP reporter (E0240), and plasmid. Ligation of promoters, reporters, and plasmids. Chris:
4A5 E/P 3395 bp
I0500 E/S (on psb2K3) 1210 bp
F2620 E/S (on pSB1A2) 1061 bp
Ryan’s Protocol for Restriction Digest Cloning Digestion (50μl reaction volume)
- Plasmid 12.0 μl
- Each enzyme 1.0 μl
- NEB buffer 5.0 μl
- 10x BSA 0 or 5 μl (optional depending on enzymes used)
- NP water Bring to 50 μl total volume (26 ul)
- Let sit for at least an hour at 37 degrees C
Heat Kill for 20 minutes at 65 degrees C
- Note: Jerome’s I0500 is extremely concentrated (1.25 ug/ul).
- Restriction Digest ran overnight until 940 am the next morning
7/7 Wednesday
Greg's Notebook
- Checked plates from previous day's transformation
- B0032 and B0014 showed little growth
- pSB1k3 showed fantastic growth, with distinct colonies covering the entire plate
- B0015, J23100, and J23107 all showed normal growth
- poured plates, stored in cold room:
- 22 Amp
- 20 Kan
- 18 Chlor
Francisco's Notebook
- Transformed E.coli with E1010 (RFP) using heatshock. Used 1uL DNA from distribution.
- Transferred 50uL of transformed cells to agar plate with kanamycin.
Chris's Notebook
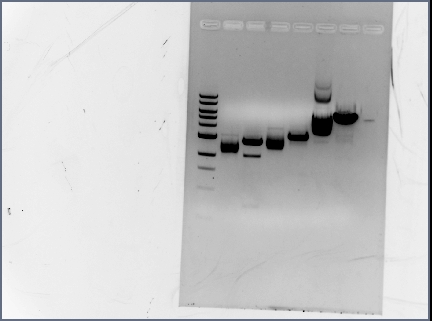
Running a Gel of F2620, pBAD, and psb4A5:
- Make a 0.8% agarose gel (0.4 g of agarose in 50 ml of TAE)
-Heat in microwave until agarose is completely dissolved -Add 10.0 ul of EtBr to mix (5000x for a 50 ml solution)
- let run for approximately 1 hr
- refer below for bands:
4A5 E/P 3395 bp
I0500 (pBAD) E/S (on psb2K3) 1210 bp
F2620 E/S (on pSB1A2) 1061 bp
Making Kanamycin, Ampicillin Chloramphenicol Plates
Kanamycin is at 1000x
PCR of I746908 to isolate the RBS, CDS, and double terminators
Programming the Block:
- 1. 95 C for 2 minutes
- 2. 95 C for 30 sec
- 3. 55 C for 30 sec
- 4. 68 C for 1.10 min
- 5. go to step 2 for 30 cycles
- 6. 72 for 5 minutes
- 7. end (0=forever)
Use PCR HF SuperMix For each reaction:
1.25 ul of forward primer 1.25 ul of reverse primer 1.0 ul of DNA template 46.5 ul of PCR HF SuperMix
7/8 Thursday
Alex's Notebook
Prepared a bunch of restriction digests. Realized some parts are way too small to follow normal BioBrick assembly.
32, 34 (X/P), J0, J7, F2620, I0500 (E/S), 1A3, 4A5, 2K3, I8, and T9 (E/P).
Christopher's Notebook
Running a Gel of F2620, pBAD, and psb4A5:
- Make a 0.8% agarose gel (0.4 g of agarose in 50 ml of TAE)
- Heat in microwave until agarose is completely dissolved
- Add 10.0 ul of EtBr to mix (5000x for a 50 ml solution)
- let run for approximately 1 hr
- refer below for bands
4A5 E/P 3395 bp
I0500 E/S (on psb2K3) 1210 bp
F2620 E/S (on pSB1A2) 1061 bp
Gel Extract the Fragments Running a Gel of PCR Product:
- first need to PCR cleanup
- then run on 1.4% agarose gel (0.7 g of agarose in 50 ml of TAE)
- this is necessary because of the 800 bp size of the fragment
Miniprep Ligations
- F2620+E0240+psb1A2
- pBAD+E0240+psb1A2
Restriction Digests PCR product F2620
Laura's Notebook
- miniprepped Alex's/Chris' liquid cultures, using Promega kit
- 2 each of:
- psb1K3
- J23107
- B0032
- eluted in 100 uL H2O
- 2 each of:
| Nanodrop Data | |||
|---|---|---|---|
| tube ID | 260/280 | 260/230 | ng/uL |
| psB1K3-1 | 1.77 | 1.08 | 71.3 |
| psB1K3-2 | 1.73 | 1.01 | 77.8 |
| J23107-1 | 1.87 | 1.60 | 116.3 |
| J23107-2 | 1.90 | 1.74 | 142.0 |
| B0032-1 | 1.81 | 1.20 | 68.1 |
| B0032-2 | 1.83 | 1.32 | 72.5 |
Greg's Notebook
- Ran nanodrop of miniprepped stuff from yesterday:
| Part # | 260/280 | 260/230 | ng/uL |
| B0034 | 1.68 | 1.65 | 26.7 |
| I746908 | 1.86 | 2.91 | 59.4 |
| J23100 | 1.01 | 2.03 | 91.4 |
| pSB3c5 | 1.81 | 2.01 | 104.8 |
Francisco's Notebook
- Toothpicked a colony from the E1010 plate from yesterday. Inoculated in 4.5mL of LB + kanamycin.
7/9 Friday
Friday Recap Meeting
- [http://docs.google.com/present/edit?id=0Ae31et9w_tAhZGZyc3R2ejJfOGNnY2drMmZz&hl=en&authkey=CPHGzfIB Weekly Leader Presentation: Karina]
- Analog Comparator update: Chris
- MDV Presentation Updates & Planning
- July 16, 2010 11:00 am
- Separate Meeting for planning, Tuesday 4:00 pm, Green Atrium
- Grad Students: Ryan
- Administrative Details
- Stipend?
- Check on Axess for Billing statements
- Sponsorship Updates
- Christina’s announcements:
- Start talking about Medal Requirements
- Keep in mind the wiki!
- Digitized Cloning Scheme
- Medal Requirements- Presentation next week how we’re addressing those
- Collaborative, Improving Standard, Human Practice
- Idea: improving promoter database on Parts Registry
- Need to get access to parts registry to edit e-mail Drew to e-mail them
- Gold Medal Meeting Meeting Wed Morning at 9:00 am? Send out the Doodle (Anusuya)
- Next Weeks Leader: Alex
Francisco's Notebook
- Miniprepped E1010 plasmids from yesterday's liquid culture.
- Nanodrop drop results: 260/280: 1.89; 260/230: 1.85; ng/uL: 282.4
Karina's Notebook
Today's Goal: Digest GFP, RFP, and Terminators. Will do (GFP + T) and (RFP + T) ligations on monday.
Part #'s:
- GFP: E0040 (cut at S and P)
- RFP: E1010 (cut at S and P)
- Term: B1006 (cut at X and P)
Recipe
- 12.0 uL DNA
- 1.0 uL each enzyme
- 5.0 uL NEB buffer 2
- 5 uL BSA (10x)
- Sterile H20 to fill up to 50 uL
Mix and put 37º waterbath for 2 or more hours.
Making Gel
To make 50 mL of 0.8% agarose gel, add:
- .4 g agarose
- 50 mL TAE (1x)
- 10 uL EtBr
Loading the Gel
- add 10 uL loading dye (6x) to digests
- add 40 uL of dye + digests to wells
- don't forget to include wells with controls! (uncut plasmids)
- load 10 uL ladder
- run at 95 V for about an hour
!!note sizes of plasmids/inserts
GFP plasmid (pSB1A2) --> 2079 bp
GFP insert --> 720 bp
RFP plasmid (pSB2K3) --> 4425 bp
RFP insert --> 681 bp
Terminator plasmid (pSB1AK3) --> 3189 bp
Terminator insert --> 39 bp
Gel Results
All bands look fine except for, there were no bands for the terminators. They were so small they ran off the gel. We are considering using PCR purification to isolate these parts.
Gel Extraction
[http://molecool.wustl.edu/krolllab/Kroll_Lab_Protocols/Molecular%20Biology%20protocols/Cloning%20protocols%20folder/Gel%20extraction-Qiagen.pdf Follow QIAquick Gel Extraction Kit Protocol], skip isopropanol step and elute in 50 uL H20.
| Gel Extraction | |||||
|---|---|---|---|---|---|
| Part | Tubes (g) | Gel Extract + tube (g) | Gel Extract (g) | ||
| GFP | 1.06 | 1.11 | .05 | ||
| RFP | 1.09 | 1.22 | .13 | ||
Laura's Lab Notebook
- worked with Karina and Francisco on digestions, gel, gel extraction (see Karina's notebook for details)
- for digestion: Francisco set up RFP, Karina set up terminator, I set up GFP
Chris's Notebook
Completion of PCR
Note: only use PCR cleanup if the DNA is going to be used for ligation
Running a Gel of PCR Product:
- first need to PCR cleanup
- then run on 1.4% agarose gel (0.7 g of agarose in 50 ml of TAE)
- this is necessary because of the 800 bp size of the fragment
- run at 85 volts
- small combs
Restriction Digest of I746908-E/P
- Cloning Digestion (50μl reaction volume):
4A5 E/P 3395 bp
I0500 E/S (on psb2K3) 1210 bp
F2620 E/S (on pSB1A2) 1061 bp
3C5 E/P 2738 bp (buffer 3)
F2620 E/S (on pSB1A2) 1061 bp
Run Gels of Restriction Digests
- run on 1.4% agarose gel (0.7 g of agarose in 50 ml of TAE)
- this is necessary because of the 800 bp size of the fragment
- run at 85 volts
- small combs
Expected Sizes:
3C5 E/P 2738 bp (buffer 3) F2620 E/S (on pSB1A2) 1061 bp I746908 E/P (on psb1A2) 2093 bp B0034 X/P (on psb1A2) 12 bp-do NOT run on gel J23100 E/S (on psb1A2) 35 bp-do NOT run on gel
Gel Extract
7/10 Saturday
Run gels for the restriction digests. 2 failed due to similar sizes Could not ligate current pieces.
New Reporter
- I746916 (N/A)
7/11 Sunday
Prepared new triple restriction digests with EcoRI, PstI, and BglI.
- I8 and T9.
Inoculated 3C5, 1K3, I0500, and I8 to obtain more DNA.
 "
"