Team:Stockholm/10 September 2010
From 2010.igem.org
(Difference between revisions)
(New page: {{Stockholm/Top2}} ==Andreas==) |
(→Andreas) |
||
| Line 1: | Line 1: | ||
{{Stockholm/Top2}} | {{Stockholm/Top2}} | ||
==Andreas== | ==Andreas== | ||
| + | ===Cloning of N-CPPs into pSB1C3 & Extraction of RBS BioBrick (BBa_B0030)=== | ||
| + | Transformations from 9/9 resulted in good colony yields on all plates. Chose "pSB1C3.N-CPP* 9/9" for colony PCR. | ||
| + | |||
| + | ====Colony PCR==== | ||
| + | Picked 12 N-CPP* clones (NC 1-12) and 4 RBS clones (RBS 1-4) | ||
| + | |||
| + | {|border="1" cellpadding="1" cellspacing="0" | ||
| + | !colspan="2"|PCR tubes | ||
| + | |- | ||
| + | |dH<sub>2</sub>O | ||
| + | |align="center" width="50"|16.22 | ||
| + | |- | ||
| + | |DreamTaq buffer | ||
| + | |align="center"|2 | ||
| + | |- | ||
| + | |dNTPs, 10 mM | ||
| + | |align="center"|0.4 | ||
| + | |- | ||
| + | |VF2 | ||
| + | |align="center"|0.4 | ||
| + | |- | ||
| + | |VR | ||
| + | |align="center"|0.4 | ||
| + | |- | ||
| + | |DreamTaq pol. | ||
| + | |align="center"|0.08 | ||
| + | |- | ||
| + | |Template DNA | ||
| + | |align="center"|0.5 | ||
| + | |- | ||
| + | | | ||
| + | !20 μl | ||
| + | |} | ||
| + | |||
| + | '''PCR settings'''<br /> | ||
| + | Standard colony PCR protocol. | ||
| + | *1:00 elongation | ||
| + | |||
| + | ====Gel verification==== | ||
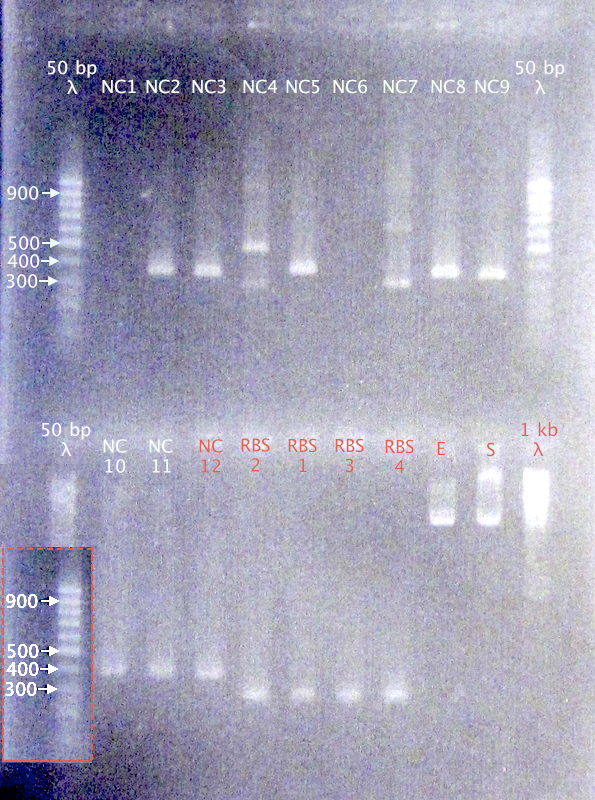
| + | [[image:ColPCR_NCPP_RBS30_10sep.png|200px|thumb|right|'''Colony PCR gel verification of pSB1C3.N-CPP (NC) and pSB1A2.BBa_B0030 (RBS) clones.'''<br />50 bp λ = GeneRuler 50 bp DNA ladder; 1 kb λ = O'GeneRuler 1 kb DNA ladder]] | ||
| + | ''Also ran two samples for Mimmi (E & S) | ||
| + | |||
| + | 1.5 % agarose, 90 V | ||
| + | |||
| + | '''Expected bands''' | ||
| + | *N-Tra10: 389 bp | ||
| + | *N-TAT: 359 bp | ||
| + | *N-LMWP: 368 bp | ||
| + | *RBS B0030: 253 bp | ||
| + | |||
| + | '''Results''' | ||
| + | *N-CPPs: Potentially correct bands for clones 2, 3, 5, 8, 9, 10, 11 & 12 | ||
| + | *RBS B0030: Correct-sized bands for all four clones. | ||
| + | |||
| + | ====ON cultures==== | ||
| + | Set ON cultures for all relevant N-CPPs, for plasmid prep. | ||
| + | *N-CPP 2, 3, 5, 8, 9, 10, 11, 12 | ||
| + | **5 ml LB + 25 Cm | ||
| + | **37 °C, 220 rpm | ||
| + | |||
| + | Selected clone 4 of RBS 30. Both plasmid prep and glycerol stock. | ||
| + | *RBS 30 4 | ||
| + | **5 ml LB + 100 Amp | ||
| + | ***37 °C, 220 rpm. | ||
| + | **3 ml LB + 100 Amp | ||
| + | ***30 °C | ||
| + | |||
| + | ===Preparation of chemically competent Top10=== | ||
| + | Since I've previously experienced slight AmpR contamination in our latest batch of competent Top10, I streaked an LB agar plate with competent Top10 cells to isolate single clones. | ||
| + | |||
| + | Plate grown ON in 37 °C. | ||
Revision as of 09:59, 13 September 2010
Contents |
Andreas
Cloning of N-CPPs into pSB1C3 & Extraction of RBS BioBrick (BBa_B0030)
Transformations from 9/9 resulted in good colony yields on all plates. Chose "pSB1C3.N-CPP* 9/9" for colony PCR.
Colony PCR
Picked 12 N-CPP* clones (NC 1-12) and 4 RBS clones (RBS 1-4)
| PCR tubes | |
|---|---|
| dH2O | 16.22 |
| DreamTaq buffer | 2 |
| dNTPs, 10 mM | 0.4 |
| VF2 | 0.4 |
| VR | 0.4 |
| DreamTaq pol. | 0.08 |
| Template DNA | 0.5 |
| 20 μl | |
PCR settings
Standard colony PCR protocol.
- 1:00 elongation
Gel verification
Also ran two samples for Mimmi (E & S)
1.5 % agarose, 90 V
Expected bands
- N-Tra10: 389 bp
- N-TAT: 359 bp
- N-LMWP: 368 bp
- RBS B0030: 253 bp
Results
- N-CPPs: Potentially correct bands for clones 2, 3, 5, 8, 9, 10, 11 & 12
- RBS B0030: Correct-sized bands for all four clones.
ON cultures
Set ON cultures for all relevant N-CPPs, for plasmid prep.
- N-CPP 2, 3, 5, 8, 9, 10, 11, 12
- 5 ml LB + 25 Cm
- 37 °C, 220 rpm
Selected clone 4 of RBS 30. Both plasmid prep and glycerol stock.
- RBS 30 4
- 5 ml LB + 100 Amp
- 37 °C, 220 rpm.
- 3 ml LB + 100 Amp
- 30 °C
- 5 ml LB + 100 Amp
Preparation of chemically competent Top10
Since I've previously experienced slight AmpR contamination in our latest batch of competent Top10, I streaked an LB agar plate with competent Top10 cells to isolate single clones.
Plate grown ON in 37 °C.
 "
"