Team:Kyoto/Notebook
From 2010.igem.org
(Difference between revisions)
| Line 1: | Line 1: | ||
{{:Team:Kyoto/Header}} | {{:Team:Kyoto/Header}} | ||
==Index== | ==Index== | ||
| - | |||
==Notebook== | ==Notebook== | ||
| - | |||
<div class="note"> | <div class="note"> | ||
===Tuesday, July 20=== | ===Tuesday, July 20=== | ||
By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto | By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto | ||
| - | |||
====1. Solubilization of antibiotics.==== | ====1. Solubilization of antibiotics.==== | ||
* for Ampicillin(Amp): add 1.0g Amp to 20ml MilliQ (final concentration is 50mg/ml). | * for Ampicillin(Amp): add 1.0g Amp to 20ml MilliQ (final concentration is 50mg/ml). | ||
| Line 13: | Line 10: | ||
* Dispense 1.1ml of the solution into 1.5ml tubes. | * Dispense 1.1ml of the solution into 1.5ml tubes. | ||
* Store in the freezer (-20℃). | * Store in the freezer (-20℃). | ||
| - | |||
====2. Making plates for LB (Amp+) and LB (Kan+).==== | ====2. Making plates for LB (Amp+) and LB (Kan+).==== | ||
| - | |||
====3. [[Team:Kyoto/Protocols#Transformation|Transformation]] of iGEM Parts.==== | ====3. [[Team:Kyoto/Protocols#Transformation|Transformation]] of iGEM Parts.==== | ||
{| class="experiments" | {| class="experiments" | ||
| Line 41: | Line 36: | ||
* And We didn't pre-culture "B0015" despite its vector is Kanamycin-resistance. | * And We didn't pre-culture "B0015" despite its vector is Kanamycin-resistance. | ||
* So, it was predicted that we will fail the transformation of "pSB4K5" and "B0015". | * So, it was predicted that we will fail the transformation of "pSB4K5" and "B0015". | ||
| + | </div> | ||
| + | |||
| + | <div class="note"> | ||
| + | ===Wednesday, July 21=== | ||
| + | By: Wataru, Ken, Makoto, Takuya Yamamoto | ||
| + | ====1. Culture plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00.==== | ||
| + | ====2. Make a master plate of the above plates.==== | ||
| + | ====3. Retry [[Team:Kyoto/Protocols#Transformation|Transformation]] of iGEM Parts.==== | ||
| + | {| class="experiments" | ||
| + | !Name||Well||Sample (µl)||Competent Cells (µl)||Total (µl)||Plate||Incubation||Result | ||
| + | |- | ||
| + | |<partinfo>pSB4K5</partinfo>||1-5-G||1||20||21||rowspan="2"|LB (Kanamycin+)||rowspan="2"|At 37℃ 7/21 20:50 - 7/22 16:30||○ | ||
| + | |- | ||
| + | |<partinfo>B0015</partinfo>||1-23-L||1||20||21||○ | ||
| + | |} | ||
| + | ====4. [[Team:Kyoto/Protocols#PCR|PCR]] for S-R-Rz/Rz1 and S==== | ||
| + | * Dilute λDNA (0.5µg/µl) 100 times with MilliQ. The final concentration of template λDNA was 5ng/µl. | ||
| + | {| class="experiments" | ||
| + | !No.||Water||25mM MgSO4||2mM dNTPs||10xBuffer for KOD Plus ver.2||TemplateDNA (5ng/µl)||Primer S-R-Rz/Rz1 Forward (10µM)||Primer S-R-Rz/Rz1 Reverse (10µM)||Primer S Reverse (10µM)||KOD Plus ver.2||Total | ||
| + | |- | ||
| + | |1||28µl||3µl||5µl||5µl||5µl||1.5µl||1.5µl||-||1µl||50µl | ||
| + | |- | ||
| + | |2||28µl||3µl||5µl||5µl||5µl||1.5µl||1.5µl||-||1µl||50µl | ||
| + | |- | ||
| + | |3||28µl||3µl||5µl||5µl||5µl||1.5µl||-||1.5µl||1µl||50µl | ||
| + | |- | ||
| + | |4||28µl||3µl||5µl||5µl||5µl||1.5µl||-||1.5µl||1µl||50µl | ||
| + | |- | ||
| + | |5||28µl||3µl||5µl||5µl||5µl||1.5µl||1.5µl||-||1µl||50µl | ||
| + | |- | ||
| + | |6||28µl||3µl||5µl||5µl||5µl||1.5µl||1.5µl||-||1µl||50µl | ||
| + | |- | ||
| + | |7||28µl||3µl||5µl||5µl||5µl||1.5µl||-||1.5µl||1µl||50µl | ||
| + | |- | ||
| + | |8||28µl||3µl||5µl||5µl||5µl||1.5µl||-||1.5µl||1µl||50µl | ||
| + | |} | ||
| + | * Forward Primer of S-R-Rz/Rz1 and S is common. | ||
| + | * PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever. | ||
| + | </div> | ||
| + | |||
| + | <div class="note"> | ||
| + | ===Thursday, July 22=== | ||
| + | By: Wataru | ||
| + | ====1. [[Team:Kyoto/Protocols#Electrophoresis|Electrophoresis]] of the PCR products for 40min.==== | ||
| + | [[Image:KyotoEXP100722-1.png|right]] | ||
| + | * '''Discussion''' | ||
| + | * Length of S and S-R-Rz/Rz1 is 370bp, 1300bp, so PCR succeeded. | ||
| + | ====2. [[Team:Kyoto/Protocols#Miniprep|Miniprep]] of iGEM Parts.==== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration(ng/µl) | ||
| + | |- | ||
| + | |<partinfo>J23100</partinfo>||18.5 | ||
| + | |- | ||
| + | |<partinfo>J23105</partinfo>||12.5 | ||
| + | |- | ||
| + | |<partinfo>J23116</partinfo>||14.6 | ||
| + | |- | ||
| + | |<partinfo>R0011</partinfo>||8.6 | ||
| + | |- | ||
| + | |<partinfo>E0840</partinfo>||12.1 | ||
| + | |- | ||
| + | |<partinfo>J06702</partinfo>||14.7 | ||
| + | |} | ||
| + | * '''Discussion''' | ||
| + | * The concentration of all samples was very week. Probably our shaking incubation was week. | ||
| + | ====3. Culture plates and make master plates of <partinfo>pSB4K5</partinfo> and <partinfo>B0015</partinfo> from 7/22 17:00 to 7/23 10:00.==== | ||
| + | </div> | ||
| + | |||
| + | <div class="note"> | ||
| + | ===Friday, July 23=== | ||
| + | By: Wataru, Tomo, Makoto | ||
| + | ====1. [[Team:Kyoto/Protocols#Miniprep|Miniprep]] of iGEM Parts.==== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration(ng/µl) | ||
| + | |- | ||
| + | |<partinfo>pSB4K5</partinfo>||79.2 | ||
| + | |- | ||
| + | |<partinfo>B0015</partinfo>||- | ||
| + | |} | ||
| + | * '''Discussion''' | ||
| + | * We lost <partinfo>B0015</partinfo> by our mistake. | ||
| + | * The concentration of <partinfo>pSB4K5</partinfo> is high, so this condition of shaking incubation is moderate. | ||
| + | ====2. Picked up 1, 3, 5, 7 of the products of PCR, and purified by PCR-purification.==== | ||
| + | {| class="experiments" | ||
| + | !Sample||Concentration (ng/µl)||New Name|| | ||
| + | |- | ||
| + | |1||18.6||- | ||
| + | |- | ||
| + | |3||77.6||S<sub>1</sub> | ||
| + | |- | ||
| + | |5||33.6||- | ||
| + | |- | ||
| + | |7||65.4||S<sub>2</sub> | ||
| + | |} | ||
| + | * '''Discussion''' | ||
| + | * The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR. | ||
| + | ====3. Retry of PCR of S-R-Rz/Rz1.==== | ||
| + | {| class="experiments" | ||
| + | !Sample||Water||25mmol/l MgSO4||2mmol/l dNTPs||10×Buffer for KOD plus ver.2||Template DNA (5ng/µl)||Primer S-R-Rz/Rz1 Forward (10µmol/l)||Primer S-R-Rz/Rz1 Reverse (10µmol/l)||KOD plus ver.2||Total | ||
| + | |- | ||
| + | |1||28µl||3||5||5||5||1.5||1.5||1||50 | ||
| + | |- | ||
| + | |2||28||3||5||5||5||1.5||1.5||1||50 | ||
| + | |- | ||
| + | |3||26.5||4.5||5||5||5||1.5||1.5||1||50 | ||
| + | |- | ||
| + | |4||26.5||4.5||5||5||5||1.5||1.5||1||50 | ||
| + | |- | ||
| + | |5||25||6||5||5||5||1.5||1.5||1||50 | ||
| + | |- | ||
| + | |6||25||6||5||5||5||1.5||1.5||1||50 | ||
| + | |} | ||
| + | * PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever. | ||
| + | ====4. Digested <partinfo>J06702</partinfo> by EcoRI, XbaI, SpeI, PstI to check function of our Restriction enzymes.==== | ||
| + | {| class="experiments" | ||
| + | !Sample||10xBuffer||BSA||Enzyme||MilliQ||Total||Incubation | ||
| + | |- | ||
| + | |1||5µl||1||''EcoR''I 0.1||3.6||10||rowspan="5"|At 37℃ 7/23 18:00 - 7/23 18:30 | ||
| + | |- | ||
| + | |2||5||1||''Xba''I 0.1||3.6||10 | ||
| + | |- | ||
| + | |3||5||1||''Spe''I 0.1||3.6||10 | ||
| + | |- | ||
| + | |4||5||1||''Pst''I 0.1||3.6||10 | ||
| + | |- | ||
| + | |5||5||1||-||3.7||10 | ||
| + | |} | ||
| + | |- | ||
| + | ====5. Electrophoresis of above sample for 35min.==== | ||
| + | [[Image:KyotoExp100723-1.png|right]] | ||
| + | * '''Discussion''' | ||
| + | * Comparison to sample 5(control, circular DNA), the bands of sample 1, 2, 3, 4 was shifted. The DNA of sample 1, 2, 3, 4 was linearized by Restriction enzymes. So, our restriction enzymes work correctly. | ||
| + | ====6. To insert S gene to GFP, we digested the PCR products of S gene by EcoRi and SpeI, and GFP by EcoRl and XbaI.==== | ||
| + | {| class="experiments" | ||
| + | !Sample||10×Buffer||Enzyme 1||Enzyme 2||MilliQ||Total||Incubation | ||
| + | |- | ||
| + | |S<sub>1</sub>||11µl||5||''EcoR''I 0.2||''Spe''I 0.2||33.6||50||rowspan="3"|At 37℃ for 2h | ||
| + | |- | ||
| + | |S<sub>2</sub>||11||5||''EcoR''I 0.2||''Spe''I 0.2||33.6||50 | ||
| + | |- | ||
| + | |<partinfo>E0840</partinfo>(GFP)||45||5||''EcoR''I 0.2||''Xba''I 0.2||0||50 | ||
| + | |} | ||
| + | * After PCR purification, evaporated them and diluted 3ul. | ||
| + | ====7. Ligated over night.==== | ||
| + | {| class="experiments" | ||
| + | !Sample||Vector||Insert||Ligation High||Total | ||
| + | |- | ||
| + | |S-GFP<sub>1</sub>||<partinfo>E0840</partinfo> 0.5µl||S<sub>1</sub> 0.5||1||2 | ||
| + | |- | ||
| + | |S-GFP<sub>2</sub>||<partinfo>E0840</partinfo> 0.5||S<sub>2</sub> 0.5||1||2 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | <div class="note"> | ||
| + | ===Monday, July 26=== | ||
| + | By: Wataru, Tomonori, Makoto | ||
| + | ====1. Electrophoresis of PCR products==== | ||
| + | [[Image:KyotoExp100726-1.png|right]] | ||
| + | * '''Discussion''' | ||
| + | * At the condition 4 (4.5µl MgSO4) and 6 (6µl MgSO4), S-R-Rz/Rz1 is amplified very much. So we decided to use them. | ||
| + | ====2. PCR purification==== | ||
| + | {| class="experiments" | ||
| + | !Sample||Concentration (ng/µl)||New Name | ||
| + | |- | ||
| + | |4||51.6|| | ||
| + | |- | ||
| + | |5||59.3|| | ||
| + | |- | ||
| + | |6||59.6|| | ||
| + | |} | ||
| + | ====3. Transformation of iGEM Parts==== | ||
| + | {| class="experiments" | ||
| + | !Name||Well||Sample (µl)||Competent Cell (µl)||Total (µl)||Plate||Incubation||Result | ||
| + | |- | ||
| + | |||1-12-M||1||20||21||LB (Amplicillin+)||rowspan="3"|At 37℃ 7/26 - 7/27||× | ||
| + | |- | ||
| + | |||2-17-F||1||20||21||rowspan="2"|LB (Kanamycin+)||× | ||
| + | |- | ||
| + | |||1-5-E||1||20||21||× | ||
| + | |} | ||
| + | ====4. Culture of 1-6-G, 1-12-O, and 1-23-L===== | ||
| + | </div> | ||
---- | ---- | ||
Revision as of 04:41, 8 September 2010
Index
Notebook
Tuesday, July 20
By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto
1. Solubilization of antibiotics.
- for Ampicillin(Amp): add 1.0g Amp to 20ml MilliQ (final concentration is 50mg/ml).
- for Kanamycin(kan): add 0.5g Kan to 10ml MilliQ (final concentration is 50mg/ml).
- Dispense 1.1ml of the solution into 1.5ml tubes.
- Store in the freezer (-20℃).
2. Making plates for LB (Amp+) and LB (Kan+).
3. Transformation of iGEM Parts.
| Name | Well | Sample (µl) | Competent Cells (µl) | Total (µl) | Plate | Incubation | Result |
|---|---|---|---|---|---|---|---|
| <partinfo>J23100</partinfo> | 1-18-C | 1 | 20 | 21 | LB (Amp+) | At 37℃ 7/20 20:50 - 7/21 17:00 | ○ |
| <partinfo>J23105</partinfo> | 1-18-M | 1 | 20 | 21 | ○ | ||
| <partinfo>J23116</partinfo> | 1-20-M | 1 | 20 | 21 | ○ | ||
| <partinfo>R0011</partinfo> | 1-6-G | 1 | 20 | 21 | ○ | ||
| <partinfo>E0840</partinfo> | 1-12-O | 1 | 20 | 21 | ○ | ||
| <partinfo>J06702</partinfo> | 2-8-E | 1 | 20 | 21 | ○ | ||
| <partinfo>pSB4K5</partinfo> | 1-5-G | 1 | 20 | 21 | × | ||
| <partinfo>B0015</partinfo> | 1-23-L | 1 | 20 | 21 | LB (Kanamycin+) | × |
- "1-18-C" means well 18C in [http://partsregistry.org/Help:Spring_2010_DNA_distribution Spring 2010 DNA Distribution Kit] Plate 1.
- Discussion
- A vector of "pSB4K5" is Kanamycin-resistance, however, we plated it to LB plate (Ampicillin+).
- And We didn't pre-culture "B0015" despite its vector is Kanamycin-resistance.
- So, it was predicted that we will fail the transformation of "pSB4K5" and "B0015".
Wednesday, July 21
By: Wataru, Ken, Makoto, Takuya Yamamoto
1. Culture plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00.
2. Make a master plate of the above plates.
3. Retry Transformation of iGEM Parts.
| Name | Well | Sample (µl) | Competent Cells (µl) | Total (µl) | Plate | Incubation | Result |
|---|---|---|---|---|---|---|---|
| <partinfo>pSB4K5</partinfo> | 1-5-G | 1 | 20 | 21 | LB (Kanamycin+) | At 37℃ 7/21 20:50 - 7/22 16:30 | ○ |
| <partinfo>B0015</partinfo> | 1-23-L | 1 | 20 | 21 | ○ |
4. PCR for S-R-Rz/Rz1 and S
- Dilute λDNA (0.5µg/µl) 100 times with MilliQ. The final concentration of template λDNA was 5ng/µl.
| No. | Water | 25mM MgSO4 | 2mM dNTPs | 10xBuffer for KOD Plus ver.2 | TemplateDNA (5ng/µl) | Primer S-R-Rz/Rz1 Forward (10µM) | Primer S-R-Rz/Rz1 Reverse (10µM) | Primer S Reverse (10µM) | KOD Plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 2 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 3 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
| 4 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
| 5 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 6 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | 1.5µl | - | 1µl | 50µl |
| 7 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
| 8 | 28µl | 3µl | 5µl | 5µl | 5µl | 1.5µl | - | 1.5µl | 1µl | 50µl |
- Forward Primer of S-R-Rz/Rz1 and S is common.
- PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever.
Thursday, July 22
By: Wataru
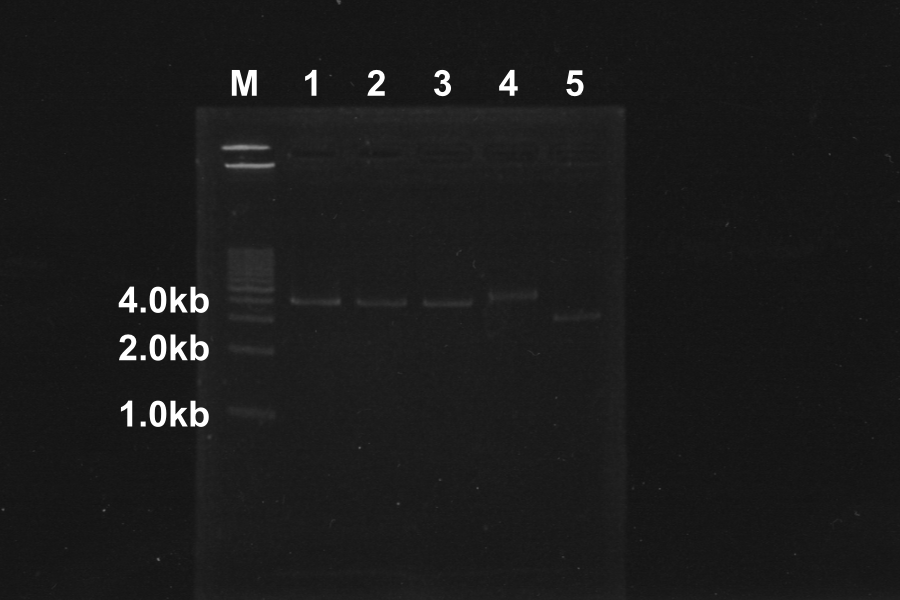
1. Electrophoresis of the PCR products for 40min.
- Discussion
- Length of S and S-R-Rz/Rz1 is 370bp, 1300bp, so PCR succeeded.
2. Miniprep of iGEM Parts.
| Name | Concentration(ng/µl) |
|---|---|
| <partinfo>J23100</partinfo> | 18.5 |
| <partinfo>J23105</partinfo> | 12.5 |
| <partinfo>J23116</partinfo> | 14.6 |
| <partinfo>R0011</partinfo> | 8.6 |
| <partinfo>E0840</partinfo> | 12.1 |
| <partinfo>J06702</partinfo> | 14.7 |
- Discussion
- The concentration of all samples was very week. Probably our shaking incubation was week.
3. Culture plates and make master plates of <partinfo>pSB4K5</partinfo> and <partinfo>B0015</partinfo> from 7/22 17:00 to 7/23 10:00.
Friday, July 23
By: Wataru, Tomo, Makoto
1. Miniprep of iGEM Parts.
| Name | Concentration(ng/µl) |
|---|---|
| <partinfo>pSB4K5</partinfo> | 79.2 |
| <partinfo>B0015</partinfo> | - |
- Discussion
- We lost <partinfo>B0015</partinfo> by our mistake.
- The concentration of <partinfo>pSB4K5</partinfo> is high, so this condition of shaking incubation is moderate.
2. Picked up 1, 3, 5, 7 of the products of PCR, and purified by PCR-purification.
| Sample | Concentration (ng/µl) | New Name | |
|---|---|---|---|
| 1 | 18.6 | - | |
| 3 | 77.6 | S1 | |
| 5 | 33.6 | - | |
| 7 | 65.4 | S2 |
- Discussion
- The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR.
3. Retry of PCR of S-R-Rz/Rz1.
| Sample | Water | 25mmol/l MgSO4 | 2mmol/l dNTPs | 10×Buffer for KOD plus ver.2 | Template DNA (5ng/µl) | Primer S-R-Rz/Rz1 Forward (10µmol/l) | Primer S-R-Rz/Rz1 Reverse (10µmol/l) | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28µl | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 2 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 3 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 4 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 5 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 6 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
- PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever.
4. Digested <partinfo>J06702</partinfo> by EcoRI, XbaI, SpeI, PstI to check function of our Restriction enzymes.
| Sample | 10xBuffer | BSA | Enzyme | MilliQ | Total | Incubation |
|---|---|---|---|---|---|---|
| 1 | 5µl | 1 | EcoRI 0.1 | 3.6 | 10 | At 37℃ 7/23 18:00 - 7/23 18:30 |
| 2 | 5 | 1 | XbaI 0.1 | 3.6 | 10 | |
| 3 | 5 | 1 | SpeI 0.1 | 3.6 | 10 | |
| 4 | 5 | 1 | PstI 0.1 | 3.6 | 10 | |
| 5 | 5 | 1 | - | 3.7 | 10 |
|-
5. Electrophoresis of above sample for 35min.
- Discussion
- Comparison to sample 5(control, circular DNA), the bands of sample 1, 2, 3, 4 was shifted. The DNA of sample 1, 2, 3, 4 was linearized by Restriction enzymes. So, our restriction enzymes work correctly.
6. To insert S gene to GFP, we digested the PCR products of S gene by EcoRi and SpeI, and GFP by EcoRl and XbaI.
| Sample | 10×Buffer | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | |
|---|---|---|---|---|---|---|---|
| S1 | 11µl | 5 | EcoRI 0.2 | SpeI 0.2 | 33.6 | 50 | At 37℃ for 2h |
| S2 | 11 | 5 | EcoRI 0.2 | SpeI 0.2 | 33.6 | 50 | |
| <partinfo>E0840</partinfo>(GFP) | 45 | 5 | EcoRI 0.2 | XbaI 0.2 | 0 | 50 |
- After PCR purification, evaporated them and diluted 3ul.
7. Ligated over night.
| Sample | Vector | Insert | Ligation High | Total |
|---|---|---|---|---|
| S-GFP1 | <partinfo>E0840</partinfo> 0.5µl | S1 0.5 | 1 | 2 |
| S-GFP2 | <partinfo>E0840</partinfo> 0.5 | S2 0.5 | 1 | 2 |
Monday, July 26
By: Wataru, Tomonori, Makoto
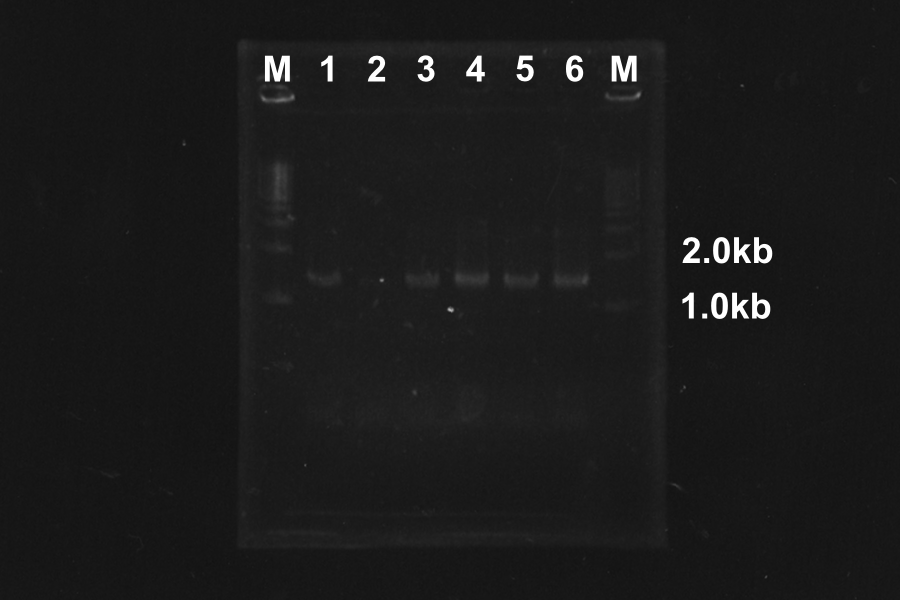
1. Electrophoresis of PCR products
- Discussion
- At the condition 4 (4.5µl MgSO4) and 6 (6µl MgSO4), S-R-Rz/Rz1 is amplified very much. So we decided to use them.
2. PCR purification
| Sample | Concentration (ng/µl) | New Name |
|---|---|---|
| 4 | 51.6 | |
| 5 | 59.3 | |
| 6 | 59.6 |
3. Transformation of iGEM Parts
| Name | Well | Sample (µl) | Competent Cell (µl) | Total (µl) | Plate | Incubation | Result |
|---|---|---|---|---|---|---|---|
| 1-12-M | 1 | 20 | 21 | LB (Amplicillin+) | At 37℃ 7/26 - 7/27 | × | |
| 2-17-F | 1 | 20 | 21 | LB (Kanamycin+) | × | ||
| 1-5-E | 1 | 20 | 21 | × |
 "
"