|
|
| (2 intermediate revisions not shown) |
| Line 122: |
Line 122: |
| | <div class="box_right"> | | <div class="box_right"> |
| | </html> | | </html> |
| - | ===76.Labortag 01.08.2010=== | + | ===6. Labday 03.05.2010=== |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Picking clones of pSB1C3_CMV</b></p>====
| + | |
| - | <p><b>Investigator: Bea</b></p>
| + | |
| - | <p style="font-size:13px; color:#66bbff;"><b>Comments:</b> Trafo was performed friday, and trafo plate were incubated over night. Clones need to be picked in order to perform Mini-Prep</p>
| + | |
| - | <ul>
| + | |
| - | <li>Bacterial strain used: XL1-B</li>
| + | |
| - | <li>Two clones were picked of trafo plate</li>
| + | |
| - | <li>Inoculating of 10 mL DYT medium containing 10µL chloramphenicol</li>
| + | |
| - | <li> Put in 37°C room on rotary shaker</li>
| + | |
| - | </ul><br>
| + | |
| - | ===77.Labortag 02.08.2010===
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Sequenzing of pSB1C3_RFC25_longlinker, pSB1C3_RFC25_SEG, pSB1C3_RFC25_GSAT </b></p>====
| + | |
| - | '''Investigator: Jessica'''<br>
| + | |
| - | <p style="font-size:13px; color:#66bbff;"><b>Comments:</b> Linkers (we got from Gerrit) we cloned in the RFC25-standard (pSB1C3_RFC25). Result looks good, linkers are in the vector pSb1C3_RFC25 <br>
| + | |
| - | <ul><li>pSB1C3_RFC25_longlinker '''P105 und P106'''</li>
| + | |
| - | <li>pSB1C3_RFC25_SEG '''P107 und P108'''</li>
| + | |
| - | <li>pSB1C3_RFC25_GSAT '''P109 und P110'''</li>
| + | |
| - | </ul>
| + | |
| | | | |
| - | </p> | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Theoretical cloning</b></p>==== |
| | | | |
| - | [[File:Freiburg10_Sequence_alignment_longlinker.jpg|500px|left|thumb|]]
| + | <p><b>Investigators: Adrian, Hanna, Bea, Patrick, Chris W. </b></p><br> |
| - | [[File:Freiburg10_Sequence_alignment_SEG.jpg|500px|left|thumb|]]
| + | |
| - | [[File:Freiburg10_Sequence_alignment_GSAT.jpg|500px|left|thumb|]]
| + | |
| - | <p style="clear:both;"> </p> | + | |
| - | <br>
| + | |
| - | <br> | + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#FF00FF;"><b>Sequencing of pGA14_Prefix-leftITR</b></p>====
| |
| - | '''Investigator: Hanna'''<br>
| |
| - | <br>
| |
| - | Eventually GATC partially managed to sequence pGA14_Prefix-left ITR clone 1 and 2. <br>
| |
| - | The sequencing results were better than before but in the ITR region still not evaluable due to the strong secondary structures. <br>
| |
| - | <br>
| |
| - | It seemed that, despite of successful insertion of the RFC10-Prefix, something went wrong because there were 2 bases missing in the NotI restriction site. But by having a closer look, it became obvious that the Geneious misinterpreted the chromatogram and overlaid the peaks of the missing bases. <br>
| |
| - | <br>
| |
| - | [[File:leftITR+Prefix.jpg|800px]]
| |
| - | <br>
| |
| - | <br>
| |
| - | For the first time sequencing of parts of the 3' end worked: The remaining NotI restriction site, which was already eliminated this week and also the "backbone-PstI" can be seen: <br>
| |
| - | <br>
| |
| - | [[File:leftITR+Prefix_2.jpg|800px]]
| |
| - | <br>
| |
| - | <br>
| |
| - | Sequencing of very GC-rich regions :) :) :) <br>
| |
| - | <br>
| |
| - | [[File:leftITR+Prefix_3.jpg|800px]]
| |
| - | <br>
| |
| - | <br>
| |
| - | Sequencing of clone 4 delivered that the ITR and the RFC10-Prefix were not inserted in the vector. The RFC25 standard of the multiple cloning site is still in the plasmid: <br>
| |
| - | <br>
| |
| - | [[File:LeftITR+Prefix clone4.jpg|800px]]
| |
| - | <br>
| |
| - | <br>
| |
| - | Therefore clone 4 was dicarded from the plasmid- and glycerol stock. <br>
| |
| - | <br>
| |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Repetition of test digestion pSB1C3_CFP SDM SspI and PvuII </b></p>====
| + | There are several matters to do in theoretical cloning. The modularization/modification of the stratagene plasmids and the enzymes. :<br> |
| - | '''Investigator: Jessica'''<br>
| + | |
| - | *buffer used: 2; Restriction-enzymes used: Enzyme 1: SspI ; Enzyme 2: PvuII
| + | |
| - | <ul><li>Plasmids (all: ~ 700 ng/µL):</li>
| + | |
| - | <ul><li>pSB1C3_CFP '''P51.1'''</li>
| + | |
| - | <li>pSB1C3_SDM_SspI '''P125'''</li>
| + | |
| - | <li>pSB1C3_SDM_PvuII '''P129'''</li>
| + | |
| - | <li>pSB1C3_SDM_PvuII '''P131'''</li></ul>
| + | |
| - | </ul>
| + | |
| - | | + | |
| - | | + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | '''Components''' ||align="left"| '''Mastermix''' ||align="left"| '''P125/µL''' ||align="left"| '''P51.1.1/µL''' ||align="left"| '''P129/µL''' ||align="left"| '''P131/µL''' ||align="left"| '''P51.1.2/µL'''
| + | |
| - | |-
| + | |
| - | | align="left" | DNA ||align="left"| -||align="left"| 2,4||align="left"| 2,3||align="left"| 1,6||align="left"| 5,3||align="left"|5,3
| + | |
| - | |-
| + | |
| - | | align="left" | BSA (10x) ||align="left"| 6||align="left"| 1||align="left"| 1||align="left"| 1||align="left"| 1||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | Buffer 2 (10x) ||align="left"| 6||align="left"| 1||align="left"| 1||align="left"| 1||align="left"| 12||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 1 SspI (68) ||align="left"|-||align="left"| 1||align="left"| 1||align="left"| -||align="left"| -||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 2 PvuII (50) ||align="left"| -||align="left"| -||align="left"| -||align="left"| 1||align="left"| 1||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | H<sub>2</sub>O ||align="left"| -||align="left"| 4,6||align="left"| 4,7||align="left"| 5,4||align="left"| 1,7||align="left"| 1,7
| + | |
| - | |-
| + | |
| - | | align="left" | '''Total volume''' ||align="left"| 12||align="left"| 10||align="left"| 10||align="left"| 10||align="left"| 10||align="left"| 10
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | | + | |
| - | *Incubation: 65 minutes<br>
| + | |
| - | '''Agarosegel'''
| + | |
| - | <br />
| + | |
| - | 0.45 g Agarose, 50 ml TEB (0,5 %), 3 µL GELRED, at 115 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | | + | |
| - | <ul><li>Marker: GeneRuler ladder mix (6x)</li></ul>
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker
| + | |
| - | !P125
| + | |
| - | !P51.1 SspI
| + | |
| - | !-
| + | |
| - | !P129
| + | |
| - | !P131
| + | |
| - | !P51.1 PvuII
| + | |
| - | | + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |7 µL
| + | |
| - | |10 µL
| + | |
| - | |10 µL
| + | |
| - | | -
| + | |
| - | |10 µL
| + | |
| - | |10 µL
| + | |
| - | |10 µL
| + | |
| - | | + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | [[File:Freiburg10_Gel test digestion SDM.jpg|500px|left|thumb|]]
| + | |
| | <br> | | <br> |
| | + | 1. Cap-Gen: |
| | + | * delete the PstI-restriction site |
| | + | * insert the antibody fragment (targeting): sequence? in which part of VP1? (Patrick) |
| | + | * add prefix & suffix |
| | + | * disable binding of Heparan Sulphat Proteoglycan |
| | <br> | | <br> |
| | + | 2. Rep-Gen: |
| | + | * delete EcoRI (2x) and PstI (2x) |
| | <br> | | <br> |
| | + | 3. ITRs: |
| | + | * NotI and PstI (in sequence?) flank the ITRs: its the question if we can/must delete them? |
| | + | * where exactly starts and ends the sequence (Patrick)? |
| | + | * it should be checked up if there is a possibility to use all of the three ITRs. |
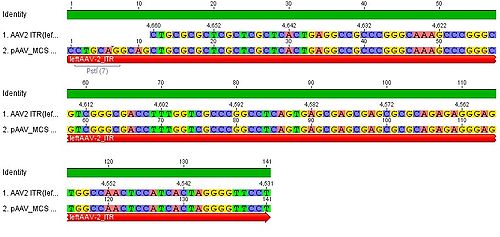
| | + | * we blasted the ITR (left) of the MCS vektor (Stratagene). we got a 92% "Coverage" with the AAV2 genom (to 100%). from the alignment (Stratagene ITR with ITR of the AAV2 genom) we got:<br> |
| | + | [[File:Freiburg10 ITR(left)-Stratagene vs. ITR AAV2.jpg|500x500px|]] |
| | + | You can see, that the ITR sequence beginns 4 bp after the PstI restriction site. thereupon we did a secundary structure analyse (www.dinamelt.bioinfo.rpi.edu), with the following structurewe got: <br> |
| | + | [[Media:Freiburg10_AAV2ITR(left)nachBlast.pdf]] <br> |
| | + | conclusion: the big loop of the secondary structure dosent change if we delete the PstI restriction site (cf. with other uploadet secondary structures), it should be considered that we can't add random bp that could affect the secundary structure. <br> |
| | + | <b>To do: which bp, which sequence could/can be insertet? cf. the genome of AAV2</b> <br> |
| | <br> | | <br> |
| | + | 4. MCS: |
| | + | * replacement through the iGEM-MCS |
| | + | * where is the beginning and ending of the MCS in the Vector? ß-globin function and sequence (search for literature and patents, which are denoted in the AAV-Helper-Free-System Manual) |
| | + | * in this context it would be also important to find out where the beta-Globulin-Intron exactly starts or rather we can cut out the MCS. |
| | <br> | | <br> |
| | + | 5. enzymes: |
| | + | * Thymidinkinase: find informations. TK30 (Bea), SR39 (Hanna) |
| | + | * Cytosindeaminase: find informations (Adrian) |
| | <br> | | <br> |
| - | <br>
| + | in addition we downloadet, addet and anotated the sequence of the pHelper plasmid of stratagene in Geneious (see "Constructs"). |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <ul><li>'''P125''' isn't cut, therefore you see the bands for supercoiled, nicked and relaxed</li>
| + | |
| - | <li>'''P51.1''' is cut once, therefore you can see a faster running band</li>
| + | |
| - | <li>'''P129''' and '''P131''' should be cut once because of the PvuII in the CFP but there is another band we can't identify</li>
| + | |
| - | <li> '''P51.1''' should be cut two times but there is also another unidentified band</li>
| + | |
| - | <ul><li>perhaps there is another PvuII in the vector we can't find (???)</li></ul>
| + | |
| - | </ul>
| + | |
| - | <p style="font-size:13px; color:#66bbff;"><b>Comments:</b> SDM of SspI is ready! SDM of PvuII looks confusing because of the lower band. P 129 will be sequenced but this doesn't solve this problem. there has to be one more rs we don't know, perhaps. we will check it...anywise</p><br>
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Mini-Prep and Test digestion of pSB1C3_CMV '''</p>====
| |
| - | '''Investigator: Kerstin''' <br>
| |
| - | '''Nanodrop'''
| |
| - | * pSB1C3_CMV clone1: 157,6 ng/µl '''P145'''
| |
| - | * pSB1C3_CMV clone2: 155,6 ng/µl '''P146'''
| |
| - | <br>
| |
| - | <br>
| |
| - | '''Test digestion''' 900ng DNA
| |
| - | {| border="1"
| |
| - | | components || align="right" |P145 /µl || align="right" |P146 /µl
| |
| - | |-
| |
| - | | DNA || align="right" | 5,7|| align="right" |5,8
| |
| - | |-
| |
| - | | BSA (10x) || align="right" |1,5 || align="right" | 1,5
| |
| - | |-
| |
| - | | Buffer 4 (10x)|| align="right" | 0,5 || align="right" | 0,5
| |
| - | |-
| |
| - | | Enzyme 1: XbaI || align="right" | 0,5 || align="right" | 1,5
| |
| - | |-
| |
| - | | Enzyme 2: PstI || align="right" | 0,5 || align="right" | 0,5
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| align="right" | 5,3|| align="right" | 5,2
| |
| - | |-
| |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | '''15'''|| align="right" | '''15'''
| |
| - | |}
| |
| - | <br>
| |
| - | agarose gel: 1,5%, digestion: 90 minutes, 37°C
| |
| - | <br>
| |
| - | [[File:Freiburg 10 pSB1C3 CMV.jpg|400px|thumb|left|]]
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| | | | |
| | + | insert the antibody fragment (targeting): sequence? in which part of VP1? (Patrick) |
| | | | |
| | + | ===7. Labday 07.05.2010=== |
| | | | |
| | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Protocolls</b></p>==== |
| | | | |
| - | '''P145 is sent for sequencing with Reverse Primer (VR2)'''
| + | <p><b>Investigators: Anissa, Kerstin </b></p><br> |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments:'''''Results look good. CMV has the expected size (~ 662bp) </p><br> | + | |
| - | '''</p>
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Sent for sequencing</b></p>====
| + | * adaption and extension of the standart prorcolls (Cloning for Pro's) |
| - | '''Investigator: Jessica'''<br> | + | * we startet to create a protokoll-mask for the praktical-cloning (short version for labwork) |
| - | '''Hanna'''<br>
| + | |
| - | <ul><li>'''P145''' left ITR</li>
| + | |
| - | <ul><li>Primer: GATC_std_pQE-FP and GATC_std_m13-FP</li></ul>
| + | |
| - | <li>'''P150''' right ITR</li>
| + | |
| - | <ul><li>Primer: GATC_std_pQE-FP and GATC_std_m13-FP</li></ul>
| + | |
| - | </ul>
| + | |
| - | <br>
| + | |
| - | '''Anissa'''<br>
| + | |
| - | <ul><li>'''P136''' pSB1C3_hgh</li>
| + | |
| - | <ul><li>Primer: O51_Reverse Primer (VR2)</li></ul>
| + | |
| - | <li>'''P139''' pAAV_BamHI</li>
| + | |
| - | <ul><li>Primer: O36_Rep_1250_rev</li></ul>
| + | |
| - | <li>'''P143''' pAAV_RC_SalI</li>
| + | |
| - | <ul><li>Primer: O53_Rep_1250_for</li></ul>
| + | |
| - | <li>'''P141''' pGL3_QTERT</li>
| + | |
| - | <ul><li>Primer: GATC_std_pTal-FP</li></ul>
| + | |
| - | <li>'''P133''' pSB1C3_betaGlobin</li>
| + | |
| - | <ul><li>Primer: O51_ReversePrimer (VR2)</li></ul>
| + | |
| - | <li>'''P145''' pSB1C3_CMV</li>
| + | |
| - | <ul><li>Primer: O51_ReversePrimer (VR2)</li></ul>
| + | |
| - | </ul>
| + | |
| - | <br>
| + | |
| - | '''Jessica'''<br>
| + | |
| - | <ul>
| + | |
| - | <li>'''P129''' pSB1C3_SDM_PvuII</li>
| + | |
| - | <ul>
| + | |
| - | <li>Primer: GATC_std_pTeSp-2</li>
| + | |
| - | </ul>
| + | |
| - | </ul>
| + | |
| - | <br>
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Preparation of competent E.coli</b></p>==== | + | ===8. Labday 10.05.2010 === |
| - | '''Investigator: Jessica'''
| + | |
| - | <ul><li>15ml DYT was prepared with 15µl tetracycline and inoculate with XL1B</li>
| + | |
| - | <li>10ml DYT w/o antibiotics was prepared and inoculate with BL21 (just for glycerol stock, no competent cells)</li></ul><br>
| + | |
| - | both were incubate over night in 37°C room
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | ====<p style="font-size:15px; background-color:#FF00FF;"><b>Last Mini-Prep and test digestion of ITRs</b></p>====
| + | |
| - | '''Investigator: Hanna'''<br>
| + | |
| - | <br>
| + | |
| - | <p style="font-size:18px; font-weight: bold; color:#FF00FF;"><u>Plasmid Mini-Prep</u></p>
| + | |
| - | *new vector name: pGA14_RFC10_leftITR and pGA14_RFC10_rightITR
| + | |
| - | <br />
| + | |
| - | <u><b>Glycerol Stocks</b></u> <br>
| + | |
| - | <b>pGA14_RFC10_leftITR</b>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| XL1blue ||align="left"| XL1blue ||align="left"| XL1blue
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pGA14_RFC10_leftITR ||align="left"| pGA14_RFC10_leftITR ||align="left"| pGA14_RFC10_leftITR
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 2.8.10 ||align="left"| 2.8.10 ||align="left"| 2.8.10
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B115 ||align="left"| B116 ||align="left"| B117
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P147 ||align="left"| P148 ||align="left"| P149
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| | | | |
| - | <b>pGA14_RFC10_rightITR</b>
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Stock solutions</b></p>==== |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| XL1blue ||align="left"| XL1blue ||align="left"| XL1blue
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pGA14_RFC10_rightITR ||align="left"| pGA14_RFC10_rightITR ||align="left"| pGA14_RFC10_rightITR
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 2.8.10 ||align="left"| 2.8.10 ||align="left"| 2.8.10
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B112 ||align="left"| B113 ||align="left"| B114
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P150 ||align="left"| P151 ||align="left"| P152
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <p style="font-size:15px; font-weight: bold; color:#FF00FF;">Test digestion</p> | + | |
| - | *buffer used: 4 ; Restriction-enzymes used: Enzyme 1 XbaI ; Enzyme 2 SpeI
| + | |
| - | *Plasmid: pGA14_RFC10_leftITR:
| + | |
| - | **Given Plasmid-Number: P147; DNA concentration: 240.22 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P148; DNA concentration: 268.97 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P149; DNA concentration: 234.16 ng/µL ;
| + | |
| - | <br>
| + | |
| - | *Plasmid: pGA14_RFC10_rightITR:
| + | |
| - | **Given Plasmid-Number: P150; DNA concentration: 221.71 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P151; DNA concentration: 191.13 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P152; DNA concentration: 215.14 ng/µL ;
| + | |
| - | <br /> | + | |
| | | | |
| - | <br /> | + | <p><b>Investigators: Adrian, Chris W., Chris L., Bea, Achim, Patrick, Hanna, (Sven)</b></p><br> |
| - | {| border="1"
| + | The following stock solutions were prepared: <br> |
| - | | align="left" | '''Components''' ||align="left"| <b>P147</b> Volume/µL ||align="left"| <b>P150</b> Volume/µL
| + | 1. Antibiotics: |
| - | |-
| + | * Ampicillin: 2 g Ampicillin were dissolved in 20 mL ethanol (70%), filled into 2 mL tubes and stored at -20°C. |
| - | | align="left" | DNA ||align="left"| 4.2 ||align="left"| 4.5
| + | * Chloramphenicol: 0.5 g Chloramphenicol were dissolved in 20 mL ethanol (70%), filled into 2 mL tubes and stored at the -20°C. |
| - | |-
| + | * Kanamycin: 1 g Kanamycin was dissolved in 20 mL multipore-H2O, sterilized by filtration and filled into 2 mL tubes and stored at the -20°C. |
| - | | align="left" | BSA (10x) ||align="left"| 1 ||align="left"| 1
| + | * Tetracyclin: 0.5 g Tetracyclin were dissolved in 20 mL ethanol (70%) and filled into 2 mL tubes. The tubes were wrapped with aluminium foil (light sensitive!) and stored at -20°C. |
| - | |-
| + | 2. ITPG solution (1 M): |
| - | | align="left" | Buffer no. 4 (10x) ||align="left"| 1 ||align="left"| 1
| + | * ~ 4.766 g ITPG were dissolved in 20 mL multipore-H2O, sterilized by filtration, filled in 2 mL tubes and stored at -20°C. |
| - | |-
| + | 3. DYT (5 litres) |
| - | | align="left" | Enzyme 1 XbaI ||align="left"| 0.5 ||align="left"| 0.5
| + | * 80 g Bactotrypton, 50 g Bactoyeast, 25 g NaCl were weight out. |
| - | |-
| + | * 2 L multipore-H2O were added |
| - | | align="left" | Enzyme 2 SpeI ||align="left"| 0.5 ||align="left"| 0.5
| + | * after mixing, multipore-H2O was added -> endvolume 5 litres |
| - | |-
| + | * medium was filled into flask and was autoclaved |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 2.8 ||align="left"| 2.5
| + | 4. Glycerol: |
| - | |-
| + | * Glycerol was filled into a flask and was then autoclaved |
| - | | align="left" | '''Total volume''' ||align="left"| <b>P147</b> ||align="left"| <b>P150</b>
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| | | | |
| - | *Incubation: 1 h
| + | '''To do: register at Mr. Gene!!!''' |
| - | <br />
| + | |
| - | <p style="font-size:15px; font-weight: bold; color:#FF00FF;">Agarose-Gel:</p>
| + | |
| - | <br />
| + | |
| - | 0.83 g Agarose, 53 mL <b>TBE</b> (1.57%), 3 µL GELRED, at 115 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample | + | |
| - | !Sample/µl] | + | |
| - | !Loading dye (5x)/µl | + | |
| - | !Expected size 1 (Geneious)
| + | |
| - | !Expected size 2 (Geneious)
| + | |
| - | |--
| + | |
| - | |P147
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |157 bp
| + | |
| - | |2902 bp
| + | |
| - | |--
| + | |
| - | |P150
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |156 bp
| + | |
| - | |2903 bp
| + | |
| - | |--
| + | |
| - | | + | |
| - | |}
| + | |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker /µL
| + | |
| - | !Sample P147 /µL
| + | |
| - | !Sample P150 /µL
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |6.5
| + | |
| - | |12
| + | |
| - | |12
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <br>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color:#FF00FF;">Test digestion delivered positive results for both ITRs: The expected fragment sizes (156 resp. 157 bp) could be detected in the referring range between the 100 and 200 bp marker nucleotides:</p> <br>
| + | |
| - | [[File:LastITRPrep.jpg|400px]] <br>
| + | |
| - | <br>
| + | |
| - | <b>Comment:</b> In order to verify the results, P147 and P150 were sent for sequencing. Because sequencing never worked before, they will be sequenced under special conditions this time.<br>
| + | |
| | <br> | | <br> |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''BioBrick production: mGMK in pSB1C3'''</p>==== | + | ===9. Labday 17.05.2010=== |
| - | Investigator: Stefan<br>
| + | |
| - | Backbone taken from: <br>
| + | |
| - | *pSB1C3_CFP (P51.2): 151,1 ng/µl<br>
| + | |
| - | Insert taken from: <br>
| + | |
| - | *pAAV_RFC25_mGMK (P100): 411,3 ng/µl<br>
| + | |
| | | | |
| | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>β-Globin</b></p>==== |
| | | | |
| - | | + | <p><b>Investigators: Bea, Chris W., Patrick, Hanna (and instructors)</b></p><br> |
| - | {| border="1"
| + | |
| - | | components || align="right" |pSB1C3_CFP(P51.2) /µl|| align="right" |pAAV_RFC25_mGMK (P100) /µl
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |8 || align="right" | 5
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |2|| align="right" | 2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" | 2
| + | |
| - | |-
| + | |
| - | |XbaI || align="right" |1 || align="right" | 1
| + | |
| - | |-
| + | |
| - | |AgeI || align="right" |1 || align="right" | 1
| + | |
| - | |-
| + | |
| - | |H<sub>2</sub>O|| align="right" |6 || align="right" | 9
| + | |
| - | |-
| + | |
| - | |'''Total volume '''|| align="right" |20 || align="right" | 20
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| | + | We blasted the sequence of the β-globin and additional nukleotides „vorne und hintendran“. |
| | + | We found only 70% coverage with the human β-globin-intron add. some parts of the exon 3. |
| | + | For this reason we think that the pAAV_MCS annotation of the β-Globin-intron of Stratagene is too generously.<br> |
| | + | In addition the alignment of the human ß-globin showed that only parts of the intron 2 (5’) and exon 3 are integratet into the vector. |
| | + | We assume that the informations from Stratagene are not total correctly ( intron flanket by the splicedonors and the acceptor-sequence). |
| | + | We blasted the sequence between the CMV-promoter and the origin beginning of the ß-globin intron. |
| | + | We found a 98,8% coverage with a synthetic CMV-promoterconstruct ( 1 nucleotide difference). |
| | + | Literature: „Diverse plasmid DNA vectors by directed molecular evolution of cytomegalovirus promoters. (Wright A. et al.)“<br> |
| | + | →The question arises if we can omit the ß-globin (because the exact function is unknown). |
| | + | For this we should contact various Companys (GeneArt, DNA2.0, Mr. Gene,…) to get more information. |
| | + | In addition we could test the expression with and without ß-globin. |
| | | | |
| - | * 1% Agarose gel
| |
| - | * 3 µl Gelred
| |
| - | *7 µl DNA-Ladder-Mix
| |
| - | * 115 Volt, running time: 50 minutes
| |
| - | Afer 50 minutes a picture (see below) was taken. Because there was no clear pattern (two bands) for the pSB1C3 backbone, the band of the insert mGMK was cut out and the gel ran another 20 minutes. After that, there were still two bands. Both were cut out and it was continued with two possible backbones. They were labeled vector (lower) and vector (upper).<br>
| |
| - |
| |
| - | [[File:Freiburg10 BioBrick pSB1C3 mGMK.jpg|400px|thumb|left|]]<br>
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | {| border="1"
| |
| - | | '''Sample/µl ''' ||''' Expected size/bp
| |
| - | |-
| |
| - | | Vector pSB1C3 || align="right" |2082
| |
| - | |-
| |
| - | | Insert mGMK || align="right" |603
| |
| - | |-
| |
| - | |}
| |
| - | <br />
| |
| - | * weight of insert mGMK gel extract: 0,17 g
| |
| - | * weight of vector (lower) pSB1C3 gel extract: 0,08 g
| |
| - | * weight of vector (upper) pSB1C3 gel extract: 0,09 g
| |
| - |
| |
| - | '''Nanodrop'''
| |
| - | * insert mGMK : 4,13 ng/µl
| |
| - | * vector (lower) pSB1C: 5,47 ng/µl
| |
| - | * vector (upper) pSB1C: 0,60 ng/µl => for calculation of ligation approach calculated with 1 ng/µl
| |
| | <br> | | <br> |
| | | | |
| - | '''Ligation'''
| + | ===10. Labortag 18.05.2010=== |
| - | *vector (lower) : insert - 4,16 µl : 4,84 µl
| + | |
| - | *vector (upper) : insert - 7,43 µl : 1,57 µl<br>
| + | |
| | | | |
| - | '''Transformation'''
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>pCMV_mVenus_YFP</b></p>==== |
| - | Two approaches were prepared, one for each vector and put in 37 °C room overnight.
| + | |
| - | <br> | + | |
| - | <br> | + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#FF00FF;"><b>Sequencing results of SDM Rep 68 & 78</b></p>====
| |
| - | '''Investigator: Hanna and Volker'''<br>
| |
| - | The sequencing results delivered that the PstI restriction sites of Rep 68 and Rep 78 were successfully deleted via side-directed mutageneis:
| |
| - | <br>
| |
| - | <gallery widths=500px heights=300px perrow=2 caption="Sequencing results of SDM Rep 68 & 78">
| |
| - | File:Rep68.jpg |Rep 68
| |
| - | File:Rep78.jpg |Rep 78
| |
| - | </gallery>
| |
| | | | |
| - | ===78.Labortag 03.08.2010===
| + | <p><b>Investigator: Bea, Chris W., Patrick, Hanna, Anissa, Kerstin, Adrian (und Instructors)</b></p> |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Results of sequencing pSB1C3_CFP_SDM_PvuII'''</p>====
| + | |
| - | '''Investigator: Jessica'''<br>
| + | |
| - | <b>SDM of PvuII:</b> | + | |
| - | [[File:PvuII.jpg|600px]]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''PvuII is succesfully deleted in the vector pSB1C3_RFC25_CFP'''
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Repetition of the quickchange site-directed mutagenesis of pAAV_RC_1.1_SalI'''</p>====
| + | |
| - | '''Investigator: Kerstin,Anissa'''<br>
| + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments:''''' Sequenzing revealed no mutagenesis of SalI in pAAV_RC_1.1_SalI, so SDM now will be repeated.</p> <br>
| + | |
| - | PCR-reaction:
| + | |
| - | {| border="1"
| + | |
| - | | '''Volume / µl''' || align="right" |'''ingredients'''|| align="right" |'''recommended /µl'''
| + | |
| - | |-
| + | |
| - | | 2,5 || align="right" |10X Pfu Ultra II buffer || align="right" | 2,5
| + | |
| - | |-
| + | |
| - | | 4,18 || align="right" |template (~10 ng)|| align="right" | 4,18 of 1:100 dilution
| + | |
| - | |-
| + | |
| - | | 0,58|| align="right" |forward primer: O68|| align="right" | 62,5 ng
| + | |
| - | |-
| + | |
| - | |0,58 || align="right" |reverse primer: O69 || align="right" | 62,5 ng
| + | |
| - | |-
| + | |
| - | |- || align="right" |DMSO || align="right" | -
| + | |
| - | |-
| + | |
| - | |0,5|| align="right" |dNTP|| align="right" | 250 µM each dNTP
| + | |
| - | |-
| + | |
| - | |16,16|| align="right" |H<sub>2</sub>O || align="right" |
| + | |
| - | |-
| + | |
| - | |0,5|| align="right" |PfuUltra II fusion (1.25) || align="right" |
| + | |
| - | |}
| + | |
| - | <br> | + | |
| - | PCR program:
| + | |
| - | {| border="1"
| + | |
| - | |'''Rounds'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time'''
| + | |
| - | |-
| + | |
| - | |1 || align="right" |95 || align="right" | 2 minutes
| + | |
| - | |-
| + | |
| - | | 20 || align="right" |95|| align="right" |30 seconds
| + | |
| - | |-
| + | |
| - | |20 || align="right" |80,4 || align="right" | 1 minute
| + | |
| - | |-
| + | |
| - | |20|| align="right" |68|| align="right" | 7,5 minutes
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | Plasmid was transformed into BL21 cells. Clones have to be picked tomorrow.
| + | |
| - | <p style="font-size:13px; color:#ff0000;">'''''Comments:'''''NO CLONES, trafo didn't work, because annealing temperature was totally to high! (80,4 °C instead of 55°C) annealing temperature should always be 55°C (in case of troubleshooting temperature can be increased up to MAX. 68°C !)</p> <br>
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''PCR for Biobrickproduction of Rep 40, 52, 68, 78 and APP'''</p>====
| |
| - |
| |
| - | '''Investigators: Volker, Anna'''<br>
| |
| - |
| |
| - |
| |
| - | Aim of the experiment:
| |
| - | We wanted to produce biobricks from the expression plasmids Rep40ex, Rep52 ex, the Rep 68 and 78 in which the PstI at position 310 was silenced and the AAP. This was performed by a PCR with primes that annealed to the ends of the later biobrick but also contained restriction sites that can be cut afterwards and cloned into pSB1C3.
| |
| - |
| |
| - | *Plasmids used as template:
| |
| - | Rep_68_ex (p119): c = 470,6 ng/µl <br>
| |
| - | Rep_78_(p122): c = 201,08 ng/µl <br>
| |
| - | Rep_40_(p22.2): c = 673,1 ng/µl <br>
| |
| - | Rep_52_(p23.2): c = 532,22 ng/µl <br>
| |
| - | pAAV-RC containing AAP ORF (p50): c = 378,5 ng/µl <br>
| |
| - |
| |
| - |
| |
| - |
| |
| - | *Primer used:
| |
| - | For Rep_40_ex: Praefix_40_52_ex & Suffix_40_68_ex<br>
| |
| - | For Rep_52_ex: Praefix_40_52_ex & Suffix_52_78_ex<br>
| |
| - | For Rep_68_ex: Praefix_68_78_ex & Suffix_40_68_ex<br>
| |
| - | For Rep_78_ex: Praefix_68_78_ex & Suffix_52_78_ex<br>
| |
| - | For AAP_ex: Praefix_AAP_ex & Suffix_AAP_ex
| |
| | | | |
| | + | Theoretical cloning with Geneious of pCMV-MCS + pGA14_mVenus_YFP --> <b>pCMV_mVenus_YFP</b> |
| | + | <ul> |
| | + | <li>Enzyme set: RFC 25 (iGEM) |
| | + | <li> digest pGA14_mVenus_YFP (insert) with XbaI and PstI: mVenus_YFP_cut_XbaI+PstI |
| | + | <li> digest pCMV_MCS (vector) with XbaI and PstI: pCMV_MCS_cut_XbaI+PstI |
| | + | <li> ligate mVenus_YFP_cut_XbaI+PstI with pCMV_MCS_cut_XbaI+PstI |
| | <br> | | <br> |
| - | *'''PCR:
| + | [[File:Freiburg10 pCMV MCS mVenus YFP.png|500x500px|]] |
| - | (was performed following the standard protocol)
| + | |
| - | <br>
| + | |
| - | {| border="1"
| + | |
| - | | '''Ingredients''' || align="right" |'''Volume / µl''' || align="right" |'''Rep68'''|| align="right" |'''Rep78''' || align="right" |'''Rep40'''|| align="right" |'''Rep52'''|| align="right" |'''AAP'''
| + | |
| - | |-
| + | |
| - | | 5X Phusion HF buffer || align="right" |10 || align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | | 10 mM dNTP mix|| align="right" |1|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | | forward primer: || align="right" |2,5|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | | reverse primer: || align="right" |2,5|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | | DNA Template|| align="right" |***|| align="right" |4,2 µl || align="right" |1 µl || align="right" |2,9 µl|| align="right" |3,8 µl|| align="right" |5,3 µl
| + | |
| - | |-
| + | |
| - | |- DMSO (2%)|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | | Phusion Polymerase|| align="right" |0,5|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |H<sub>2</sub>O|| align="right" |*** || align="right" | 28,3 µl|| align="right" | 31,5 µl|| align="right" | 29,6 µl|| align="right" | 28,7 µl|| align="right" |27,2 µl
| + | |
| - | |-
| + | |
| - | |Total volume|| align="right" |50
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | | + | |
| - | PCR program:
| + | |
| - | {| border="1"
| + | |
| - | |'''PCR Program'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time''' || align="right" |'''Rep40'''|| align="right" |'''Rep50'''|| align="right" |'''Rep68'''|| align="right" |'''Rep78'''|| align="right" |'''AAP'''
| + | |
| - | |-
| + | |
| - | |1|| align="right" |98 || align="right" |1min|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |2|| align="right" |98 || align="right" |15s|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |8x|| align="right" |*** || align="right" |25s|| align="right" |63°C|| align="right" |62°C|| align="right" |63°C|| align="right" |62°C|| align="right" |64°C
| + | |
| - | |-
| + | |
| - | |3 || align="right" |72|| align="right" |***|| align="right" |15s || align="right" |18s|| align="right" |24s || align="right" |27s || align="right" | 10s
| + | |
| - | |-
| + | |
| - | |4 || align="right" |98|| align="right" | 15s|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |17x|| align="right" |***|| align="right" |25s|| align="right" |68°C|| align="right" |66°C|| align="right" |68°C|| align="right" |64°C|| align="right" |68°C
| + | |
| - | |-
| + | |
| - | |5|| align="right" |72|| align="right" |***|| align="right" |15s|| align="right" |18s|| align="right" |24s|| align="right" |27s|| align="right" |10s
| + | |
| - | |-
| + | |
| - | |6x|| align="right" |72|| align="right" |5min|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |Hold|| align="right" |4|| align="right" | || align="right" | || align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | | + | |
| - | Five µl of PCR-product were used for an analytical gel to see if the PCR worked. <br>
| + | |
| - | | + | |
| - | <gallery widths=500px heights=300px perrow=2 caption="BioBrick production for Rep and AAP">
| + | |
| - | Image:Freiburg10_Biobrick-production for Rep40-78ex and AAPex.jpg|Alalytical gel
| + | |
| - | Image:Freiburg10_Biobrick-production for Rep40-78ex and AAPex praeparative gel.jpg|Praeparative gel
| + | |
| - | </gallery>
| + | |
| - | | + | |
| - | <p style="color:red;">Comment: The result is that the PCR worked for the longer Rep proteins (68&78) with which a Quick-change was performed to remove the PstI(310) but not with the constructs for the smaller Rep variants (40&52) that were recieved from PD Kleinschmidt. As the longer Rep variants the AAP PCR Reaction also resulted in a PCR-product of the expected size. Unfortunately there were a secondary band of ~90bp for Rep_68_ex. For this reason we performed a praeparative gel and decided to cut the bands of all PCR products.</p> <br>
| + | |
| - | | + | |
| - | The bands marked in the gel picture were cut and a gel extraction was performed.
| + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Gel extraction</p>
| + | |
| - | <br />
| + | |
| - | Gel measurement:
| + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | '''Sample'''
| + | |
| - | | align="left" | '''Weight'''
| + | |
| - | | align="left" | '''Volume'''
| + | |
| - | | align="left" | '''Concentration'''
| + | |
| - | |-
| + | |
| - | | align="left" | Rep_68_ex
| + | |
| - | | align="left" | 0,1 g
| + | |
| - | | align="left" | 20 µl
| + | |
| - | | align="left" | 17,6 ng/µl
| + | |
| - | |-
| + | |
| - | | align="left" | Rep_78_ex
| + | |
| - | | align="left" | 0,15g
| + | |
| - | | align="left" | 20 µl
| + | |
| - | | align="left" | 80,6 ng/µl
| + | |
| - | |-
| + | |
| - | | align="left" | AAP_ex
| + | |
| - | | align="left" | 0,19 g
| + | |
| - | | align="left" | 20µl
| + | |
| - | | align="left" | 77,78 ng/µl
| + | |
| - | |}<br>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Continuation of preparation of competent E.coli</b></p>====
| + | |
| - | '''Investigator: Jessica'''<br>
| + | |
| - | preparation of competent XL1blue was finished according to the standard protocol
| + | |
| - | <ul><li>aliquots of 60µl (to use for 1 trafo) are stored in -80°C freezer</li></ul>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Sequencing of pSB1C3</b></p>====
| + | |
| - | '''Investigator: Jessica'''<br>
| + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments:''''' We sent for sequencing '''pSB1C3_RFC25_longlinker (P105)''' to check the strange result of the test digestion of pSB1C3_SDM_PvuII(01.08.10. <br>
| + | |
| - | pSB1C3_SDM_PvuII is too long for sequencing wherefore we sent pSB1C3_RFC25_longlinker because the longlinker just have 54kb (bp, nicht kb (Volker) (necessary is just the backbone we don't have.</p>
| + | |
| - | <ul><li>Primer:</li>
| + | |
| - | <ul>
| + | |
| - | <li>RESgen-241698</li>
| + | |
| - | <li>Reverse primer (VR2)</li>
| + | |
| - | <li>o_F1-fw (from Gerrit)</li>
| + | |
| | </ul> | | </ul> |
| - | </ul>
| |
| - | <br>
| |
| - | <br>
| |
| | | | |
| - | ====<p style="font-size:15px; background-color:#FF00FF;"><b>Design of Primer for p5 Promoter WT and TATA-less BioBrick production</b></p>==== | + | ===11. Labortag 19.05.2010=== |
| - | '''Investigator: Hanna'''<br>
| + | |
| - | <br>
| + | |
| - | [[File:Freiburg10 p5 Primer.JPG|500px|thumb|left|]]
| + | |
| - | <br>
| + | |
| | | | |
| - | [[File:Freiburg10 p5Primer Aim.JPG|500px|thumb|right|]]
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Cloning of pCMV-MCS + pGA14_mVenus_YFP --> <b>pCMV_mVenus_YFP</b></p>==== |
| - | <br>
| + | |
| - | <br> | + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br> | + | |
| - | <br> | + | |
| - | <br> | + | |
| | | | |
| - | ===79.Labortag 04.08.2010===
| + | <p><b>Investigators: Adrian, Bea, Chris W., Hanna, Patrick</b></p> |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Cloning of right ITR and left ITR into pSB1C3_RFC25_CFP'''</p>====
| + | <b>Digestion</b> |
| - | Intention: Get biobricks ready. <br>
| + | |
| - | Investigator: Patrick <br>
| + | |
| - | pGA14_lITR_RFC10 (P147), pGA14_rightITR (P150) and pSB1C3_RFC25_CFP (P51.1) were digested with EcoRI and PstI (Buffer 4) according to the standard protocol. Digestion Time: 80 minutes.
| + | |
| | <br> | | <br> |
| - | GelRun: 1% Agarose Gel. Expected results (from left to right):
| + | <ul> |
| - | *pSB1C3_RFC25_CFP: about 2100 bp and 800 bp.
| + | <li>plasmid: insert: pGA14_mVenus_YFP; number: P1 production date: ____ origin: ____ </li> |
| - | *pGA14_leftITR_RFC10: the size of the insert should be 135 bp.
| + | <li>plasmid: vector: pCMV_MCS; number: P2 production date: ____ origin: ____ </li> |
| - | *pGA14_rightITR_RFC10: the size of the insert should be 138bp.
| + | <li>new vector name: pCMV_mVenus_YFP <br></li> |
| - | [[File:20100704_patrick_bearbeitet.JPG|400px]]<br>
| + | <li>buffer used:3 ; Restriction-enzymes used: Enzyme XbaI (no. Lab:___) ; Enzyme PstI(no.Lab:___)</li> |
| - | <br> | + | <li>DNA concentration (vector): 375 ng/µl ; DNA concentration (insert): 476 ng/µl</li> |
| - | The mutual vector (now without CFP) and inserts (left ITR and right ITR) were cut out. The gelextraction was performed according to the standard protocol. DNA concentration of the extracts:
| + | |
| - | * pSB1C3_RFC25_CFP: 2,9 ng/µl
| + | |
| - | * left ITR: 1,4 ng/µl
| + | |
| - | * right ITR: 2,0 ng/µl
| + | |
| - | <br> | + | |
| - | The Quick Ligation was not performed according to the standard protocol:
| + | |
| - | * left ITR + pSB1C3: 5µl Buffer, 1 µl Quick-Ligase, 2,5 µl left ITR, 1,5 µl pSB1C3_RFC25.
| + | |
| - | * right ITR + pSB1C3: 5µl Buffer, 1 µl Quick-Ligase, 2,5 µl right ITR, 1,5 µl pSB1C3_RFC25.
| + | |
| - | | + | |
| - | <br> | + | |
| - | Transformation: performed according to the standard protocol (BL21). The cells were plated on a agar plate with chloramphenicol. The clones will be picked tomorrow.
| + | |
| - | <br> | + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Cloning of SDM SspI and SDM PvuII'''</p>====
| + | |
| - | '''Investigator: Jessica'''
| + | |
| - | *Vector: name: pSB1C3_SDM_SspI '''P125'''
| + | |
| - | *Insert: name: pSB1C3_SDM_PvuII '''P129'''
| + | |
| - | *new vector name: pSB1c3_SDM_SspI/PvuII '''P157'''
| + | |
| - | *buffer used: 4 ; Restriction-enzymes used: Enzyme 1 (no. Lab: 141) BstI ; Enzyme 2 (no.Lab: 144) BpmI
| + | |
| - | *DNA concentration (vector): 321,9 ng/µl ; DNA concentration (insert): 308,4 µg/µl
| + | |
| | | | |
| - | <br />
| |
| | {| border="1" | | {| border="1" |
| - | | '''components''' || align="right" |'''volume of pSB1C3_SDM_SspI /µl''' || align="right" |'''volume of pSB1C3_SDm_PvuII /µl''' | + | | components || align="right" | V (pGA_mVenus_YFP)/ µl ||align="right"| V(pCMV_MCS) / µl |
| | |- | | |- |
| - | | DNA || align="right" |4,66 || align="right" |4,87 | + | | DNA || align="right" | 4||align="right"|2,7 |
| | |- | | |- |
| - | | BSA (10x) || align="right" |2 || align="right" | 2 | + | | BSA (10x) || align="right" |2||align="right"|2 |
| | |- | | |- |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2 | + | | Buffer 3 (10x)|| align="right" |2||align="right"|2 |
| | |- | | |- |
| - | |Enzyme BstI (no.Lab:141)|| align="right" |1 || align="right" |1 | + | |Enzyme: XbaI (no.Lab:___)|| align="right" |1,5||align="right"|1,5 |
| | |- | | |- |
| - | |Enzyme BpmI (no.Lab:144)|| align="right" |1 || align="right" |1 | + | |Enzyme: PstI (no.Lab:___)|| align="right" |1||align="right"|1 |
| | |- | | |- |
| - | |H2O|| align="right" |9,34 || align="right" |9,13 | + | |H2O|| align="right" |9,5||align="right"|10,8 |
| | |- | | |- |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 20|| align="right" |20 | + | |'''Total volume'''|| align="right" |<b>20</b>||align="right"|<b>20</b> |
| | |} | | |} |
| | | | |
| - | <br /> | + | <li> Incubation: 1 h at 37°C</li> |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 130 Volt, running time:45
| + | </ul> |
| - | <br /> | + | |
| - | <br /> | + | |
| | | | |
| - | <br />
| + | <b>1% Agarose gel and Gel extraction</b> |
| - | '''Loading plan for agarose gel''': <br>
| + | |
| - | Marker used: GeneRuler ladder mix (Fermentas)<br>
| + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker
| + | |
| - | !Sample 125, 20µl
| + | |
| - | !Sample 129, 20µl
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |1
| + | |
| - | |3
| + | |
| - | |5
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | '''Results''': digesttemperature of BstI is 55°C, plasmid was just digested at 37°C... :( (<br>
| + | |
| - | [[File:Digestion of SDM SspI and SDM PvuII.jpg|400px]] <br>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''2nd Repetition of Quickchange site-directed mutagenesis of pAAV_RC_1.1_SalI'''</p>====
| + | |
| - | '''Investigator: Kerstin, Anissa'''<br>
| + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments:''''' Last trafo didn't work. Annealing temperature was to high (80,4°C instead of 55°C) </p> <br>
| + | |
| - | | + | |
| - | Two approaches were made (short and long PCR:
| + | |
| - |
| + | |
| - | PCR-reaction:
| + | |
| - | {| border="1"
| + | |
| - | | '''Volume / µl''' || align="right" |'''ingredients'''|| align="right" |'''recommended /µl'''
| + | |
| - | |-
| + | |
| - | | 2,5 || align="right" |10X Pfu Ultra II buffer || align="right" | 2,5
| + | |
| - | |-
| + | |
| - | | 1,83 || align="right" |template (~10 ng): p139 (c: 546,97 ng/µl)|| align="right" | 1,83µl of 1:100 dilution
| + | |
| - | |-
| + | |
| - | | 0,58|| align="right" |forward primer: O68|| align="right" | 62,5 ng
| + | |
| - | |-
| + | |
| - | |0,58 || align="right" |reverse primer: O69 || align="right" | 62,5 ng
| + | |
| - | |-
| + | |
| - | |- || align="right" |DMSO || align="right" | -
| + | |
| - | |-
| + | |
| - | |0,5|| align="right" |dNTP|| align="right" | 250 µM each dNTP
| + | |
| - | |-
| + | |
| - | |18,51|| align="right" |H<sub>2</sub>O || align="right" |
| + | |
| - | |-
| + | |
| - | |0,5|| align="right" |PfuUltra II fusion (1.25) || align="right" |
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | PCR program (long):
| + | |
| - | {| border="1"
| + | |
| - | |'''Cycles'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time'''
| + | |
| - | |-
| + | |
| - | |1 || align="right" |95 || align="right" | 2 minutes
| + | |
| - | |-
| + | |
| - | | 20 || align="right" |95|| align="right" |30 seconds
| + | |
| - | |-
| + | |
| - | |20 || align="right" |55 || align="right" | 1 minute
| + | |
| - | |-
| + | |
| - | |20|| align="right" |68|| align="right" | 7,5 minutes
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | PCR program (short):
| + | |
| - | {| border="1"
| + | |
| - | |'''Cycles'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time'''
| + | |
| - | |-
| + | |
| - | |1 || align="right" |95 || align="right" | 2 minutes
| + | |
| - | |-
| + | |
| - | | 20 || align="right" |95|| align="right" |30 seconds
| + | |
| - | |-
| + | |
| - | |20 || align="right" |55 || align="right" | 1 minute
| + | |
| - | |-
| + | |
| - | |20|| align="right" |68|| align="right" | 4 minutes
| + | |
| - | |}
| + | |
| - | Plasmids were transformed into BL21 cells. Clones have to be picked tomorrow.
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep of pSB1C3_mGMK</b></p>====
| + | |
| - | <b>Investigator: Chris W., Bea</b><br>
| + | |
| - | | + | |
| - | | + | |
| - | Vector name: pSB1C3_mGMK upper band 1.1 and pSB1C3_mGMK upper band 1.2 and pSB1C3_mGMK lower band 2.1 and pSB1C3_mGMK lower band 2.2
| + | |
| - | | + | |
| - | Mini-Prep following the standart Protokoll
| + | |
| - | <br />
| + | |
| - | <li>P153 = pSB1C3_mGMK upper band 1.1 = 248,3 ng/µl
| + | |
| - | <li>P154 = pSB1C3_mGMK upper band 1.2 = 251,6 ng/µl
| + | |
| - | <li>P155 = pSB1C3_mGMK lower band 1.1 = 263 ng/µl
| + | |
| - | <li>P156 = pSB1C3_mGMK lower band 1.2 = 235,2 ng/µl
| + | |
| - | <br />
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <b>Test digestion:</b>
| + | |
| | <ul> | | <ul> |
| - | <li>Perfomed with 15 µL of total volume with all four clones</li> | + | <li>prepare 1% agarose gel, run gel for 45 minutes(119 V)</li> |
| - | <table border=1 cellpadding=0 cellspacing=0 width=621 style='border-collapse:
| + | <li>cut out insert and vector</li> |
| - | collapse;table-layout:fixed;width:466pt'>
| + | <li>perform gel extraction following standard protocol provided by Qiagen</li> |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt'>
| + | |
| - | <td height=29 class=xl6724191 width=143 style='height:21.75pt;width:107pt'> </td>
| + | |
| - | <td class=xl6824191 width=131 style='border-left:none;width:98pt'>Mastermix/µL</td>
| + | |
| - | <td class=xl6824191 width=87 style='border-left:none;width:65pt'>P153/µL</td>
| + | |
| - | <td class=xl6824191 width=84 style='border-left:none;width:63pt'>P154/µL</td>
| + | |
| - | <td class=xl6824191 width=90 style='border-left:none;width:68pt'>P155/µL</td>
| + | |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P156/µL</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>DNA</span></td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>-</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,5</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,5</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,5</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,5</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| + | |
| - | | + | |
| - | 1'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>BSA (10x)</span></td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>7,5</td>
| + | |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>4</td>
| + | |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>4</td>
| + | |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>4</td>
| + | |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>4</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| + | |
| - | | + | |
| - | 2'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>Buffer
| + | |
| - | 4<span style='mso-spacerun:yes'> </span>(10x)</span></td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>7,5</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| + | |
| - | | + | |
| - | 3'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>Enzyme
| + | |
| - | XbaI<span style='mso-spacerun:yes'> </span></span></td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,5</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| + | |
| - | | + | |
| - | 4'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>Enzyme
| + | |
| - | AgeI</span></td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,5</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| + | |
| - | | + | |
| - | 5;mso-yfti-lastrow:yes'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>H2O</span></td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>-</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>7,5</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>7,5</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>7,5</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>7,5</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt'>
| + | |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| + | |
| - | | + | |
| - | none;width:107pt'>Total volume</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>15</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>15</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>15</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>15</td>
| + | |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>15</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=0 style='display:none'>
| + | |
| - | <td width=143 style='width:107pt'></td>
| + | |
| - | <td width=131 style='width:98pt'></td>
| + | |
| - | <td width=87 style='width:65pt'></td>
| + | |
| - | <td width=84 style='width:63pt'></td>
| + | |
| - | <td width=90 style='width:68pt'></td>
| + | |
| - | <td width=86 style='width:65pt'></td>
| + | |
| - | </tr>
| + | |
| - | </table>
| + | |
| - | <br />
| + | |
| - | </ul>
| + | |
| - | <ul>
| + | |
| - | <li>All four samples were loaded on a 1% agarose gel</li>
| + | |
| - | <li>P153 = pSB1C3_mGMK upper band 1.1 </li> | + | |
| - | <li>P154 = pSB1C3_mGMK upper band 1.2 </li> | + | |
| - | <li>P155 = pSB1C3_mGMK lower band 1.1</li>
| + | |
| - | <li>P156 = pSB1C3_mGMK lower band 1.2</li>
| + | |
| - | </ul>
| + | |
| - | <br />
| + | |
| - | [[File:Freiburg10 04 08 2010 Test digestion of pSB1C3 mGMK.jpg|400px]]
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <b> Sequencing</b>:
| + | |
| - | <ul>
| + | |
| - | <li> Plasmid used: P156: pSB1C3_mGMK lower band 1.2</li>
| + | |
| - | <li> Primer used: VR-2</li>
| + | |
| - | <li> Tube name: Bea_1</li>
| + | |
| | </ul> | | </ul> |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">''' cell culture '''</p>====
| + | <b>Ligation</b> |
| | | | |
| - | Investigator: Adrian
| |
| - | <br />
| |
| - | <b> Plan for the next virus production procedures </b>
| |
| - | <br />
| |
| - | The Motivation: investigation of the influence of different GOI amounts.
| |
| - | <br />
| |
| - | The Plan: keep rep/cap and pHelper amount stable (3,3 µg each => 6,6 µg), differ the GOI (YFP) amount in each stock.
| |
| - | <br />
| |
| - | Viral Stocks:
| |
| - | * 3,3 µg YFP + 3,3 µg pHelper + 3,3 rep/cap
| |
| - | * 10 µg YFP + 3,3 µg pHelper + 3,3 rep/cap
| |
| - | * 20 µg YFP + 3,3 µg pHelper + 3,3 rep/cap
| |
| - | <br />
| |
| - | *3,3 µg pHelper + 3,3 rep/cap (<b>without GOI !!!</b>)
| |
| - | <br />
| |
| - | *one GMK_TK clone (with the confirmed sequence) + 3,3 µg pHelper + 3,3 rep/cap
| |
| - | <br />
| |
| - |
| |
| - | <b>Transduction plan</b>
| |
| - |
| |
| - | <li>five 6-well-plates wille be transduced
| |
| - | {| align=right
| |
| - | |}
| |
| - | {| border="1"
| |
| - | |A
| |
| - | !150µl AAV stock 1
| |
| - | !300µl AAV stock 1
| |
| - | !control no Virus
| |
| - | |-
| |
| - | !B
| |
| - | |150µl AAV stock 2
| |
| - | |300µl AAV stock 2
| |
| - | |150µl AAV without GOI
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Repetition of cloning of SDM SspI and SDM PvuII'''</p>====
| |
| - | '''Investigator: Jessica'''
| |
| - | *Vector: name: pSB1C3_SDM_SspI '''P125'''
| |
| - | *Insert: name: pSB1C3_SDM_PvuII '''P129'''
| |
| - | *new vector name: pSB1C3_RFC25_SDM_SspI/PvuII '''P157'''
| |
| - | *buffer used: 4 ; Restriction-enzymes used: Enzyme 1 (no. Lab: 141) BtsI ; Enzyme 2 (no.Lab: 144) BpmI
| |
| - | *DNA concentration (vector): 321,9 ng/µl ; DNA concentration (insert): 308,4 µg/µl
| |
| - |
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | '''components''' || align="right" |'''volume of pSB1C3_SDM_SspI /µl''' || align="right" |'''volume of pSB1C3_SDm_PvuII /µl'''
| |
| - | |-
| |
| - | | DNA || align="right" |4,66 || align="right" |4,87
| |
| - | |-
| |
| - | | BSA (10x) || align="right" |2 || align="right" | 2
| |
| - | |-
| |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2
| |
| - | |-
| |
| - | |Enzyme ClaI (no.Lab:152)|| align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | |Enzyme BpmI (no.Lab:144)|| align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | |H2O|| align="right" |9,34 || align="right" |9,13
| |
| - | |-
| |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 20|| align="right" |20
| |
| - | |}
| |
| - | incubation time: 1,5h
| |
| - | <br />
| |
| - | 0,5 g Agarose,50 ml TBE (1%), 3 µl GELRED (gel was shared with Anna) , at Volt, running time:
| |
| - | <br />
| |
| - | <br />
| |
| - |
| |
| - | <br />
| |
| - | '''Loading plan for agarose gel''': <br>
| |
| - | Marker used: GeneRuler ladder mix (Fermentas)<br>
| |
| - |
| |
| - | {| border="1"
| |
| - | |
| |
| - | !Marker
| |
| - | !Sample 125, 20µl
| |
| - | !Sample 129, 20µl
| |
| - | |-
| |
| - | !Lane
| |
| - | |1
| |
| - | |3
| |
| - | |5
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | This appproach also didn't work, same result. the guess is that BpmI doesn't work anymore. will be repeated by Chris W. on 05.08.10. the result will show that the guess is right and BpmI from labstock is changed out with a new one.
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Test transformation of XL1 competent cells'''</p>====
| |
| - | Investigator: Kira
| |
| - |
| |
| - | Test transformation was performed in order to test the efficiency of produced chemical competent XL1 blue cells.
| |
| - |
| |
| - | 2 plates were prepared: one with 50 pg and the second with 100 pg pUC 18 plasmid (50 pg/㎕)
| |
| - | 50 pg plate: 1 ㎕ pUC + 50 ㎕ XL1B cells
| |
| - | 100 pg plate: 2 ㎕ pUC + 50 ㎕ XL1B cells
| |
| - |
| |
| - | Transformation was performed according to the standard protocol and the plates were incubated at 37 C.
| |
| - |
| |
| - | <br/>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#ff00ff;">'''Sequencing results of ITRs'''</p>====
| |
| - | Investigator: <b>Hanna</b>
| |
| - | <br/>
| |
| - | 8 sequencing files were analyzed "per hand" base by base. Alignments (will be inserted soon) delivered that the right ITR is 100% OK and was successfully converted into the RFC10 BioBrick standard. <br/>
| |
| - |
| |
| - | [[File:Freiburg10_Sequencing_RFC10RightITR_2.jpg]] <br/>
| |
| - |
| |
| - | The sequencing and alignments of the left ITR delivered that a 15 bp fragment in the middle of the sequence is lacking. Therefore further test digestions have to be performed. Nevertheless also this ITR was successfully converted into the RFC10 BioBrick standard. Secondary structure analysis showed that the stemloop-structure will be nevertheless forming. We will try to test whether the referring fragment is also missing in the pAAV_MCS. If it's also lacking there we will continue with this ITR and test whether it functions.
| |
| - | <br/>
| |
| - | [[File:Freiburg10 Sequencing RFC10LeftITR 2.jpg]]
| |
| - | <br/>
| |
| - | <br/>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Cloning of Rep68, Rep78 and AAP into pSB1C3'''</p>====
| |
| - | '''Investigator: Anna
| |
| - |
| |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': The PCR of Rep 40/52 didn't work (see 03.08), whereas the PCR of Rep 68/78 and AAP was succesful. Ligation was done with two different samples of pSB1C3 (see agarose gel). Samples from digestion and from ligation are stored in the 4°C freezer.</p> <br>
| |
| - |
| |
| - | *Digestion of PCR products and vector:
| |
| - |
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | '''components''' || align="right" |'''PCR product /µl''' || align="right" |'''vector /µl'''
| |
| - | |-
| |
| - | | DNA || align="right" |19 || align="right" |10
| |
| - | |-
| |
| - | | BSA (10x) || align="right" |3 || align="right" | 3
| |
| - | |-
| |
| - | | Buffer 4 (10x)|| align="right" |3 || align="right" |3
| |
| - | |-
| |
| - | |Enzyme '''XbaI || align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | |Enzyme '''SpeI || align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | |H2O|| align="right" |3 || align="right" |12
| |
| - | |-
| |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 30|| align="right" |30
| |
| - | |}
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': Digestion was done with XbaI and SpeI, it has to be checked if the inserts are cloned into the vector in the right orientation. </p> <br>
| |
| - |
| |
| - | *Purification of Rep68, 78 and AAP:
| |
| - | For the purification 95 µl of buffer PBI was used.
| |
| - |
| |
| - | c(Rep68)= 17,6 ng/µl <br />
| |
| - | c(Rep78)= 80,60 ng/µl <br />
| |
| - | c(AAP)= 77,78 ng/µl
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | *Gelextraction of pSB1C3_RFC25_CFP:
| |
| - | 0,5 g Agarose,50 ml TBE (1%), 3 µl GELRED , at 115 Volt, running time:55 <br />
| |
| - | 5µl loading dye (6x) for the sample, Marker: GeneRuler ladder mix (Fermentas)
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - |
| |
| - | [[file:Digestion of pSB1C3.jpg]]
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - | c(pSB1C3)= 2,87 ng/µl <br />
| |
| - | c(pSB1C3_2)= 9,46 ng/µl
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | *Quickligation of PCR products and vector:
| |
| - |
| |
| - | For the Ligation 10µl buffer (2x) and 1µl Quickligase were used.
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | ''' ''' || align="right" |'''Sample-no.'''|| align="right" |'''vector /µl''' || align="right" |'''insert /µl'''
| |
| - | |-
| |
| - | | pSB1C3 + Rep68 || align="right" |1.1 || align="right" |6,51 || align="right" |2,4
| |
| - | |-
| |
| - | | pSB1C3_2 + Rep68 || align="right" |1.2 || align="right" |3,9 || align="right" | 5,02
| |
| - | |-
| |
| - | | pSB1C32 + Rep78 || align="right" |2.1 || align="right" |8,2 || align="right" |0,8
| |
| - | |-
| |
| - | | pSB1C3_2 + Rep78 || align="right" |2.2 || align="right" |6,82 || align="right" |2,18
| |
| - | |-
| |
| - | | pSB1C3 + AAP || align="right" |3.1 || align="right" |8,71 || align="right" |0,92
| |
| - | |-
| |
| - | | pSB1C3_2 + AAP || align="right" |3.2 || align="right" |8,1 || align="right" |0,9
| |
| - | |-
| |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 9|| align="right" | || align="right" |
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | *Transformation:
| |
| - |
| |
| - | The transformation was done following the standard protocol using B21 cells.
| |
| - |
| |
| - | ===80.Labortag 05.08.2010===
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>New LB Agar was prepared.</b></p>====
| |
| - | Investigator: Patrick
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Sequenc analysis of BioBricks: CMV, hGH and beta globin</b></p>====
| |
| - | <b>Investigator: Bea</b><br />
| |
| - | <p style="font-size:13px; color:#68bbff;"><b>Comments</b>: Sequence analysis of three BioBricks which were cloned into the iGEM standard plasmid pSB1C3. Cloning of this three plasmids were performed at: </p> <br/ >
| |
| | <ul> | | <ul> |
| - | <li> </li> | + | <li>Measure DNA-concentration with Nanodrop </li> |
| - | <li> </li> | + | <li>c(mVenus_YFP) = 16,8 ng/µL</li> |
| - | <li> </li> | + | <li>c(pCMV_MCS) = 22,8 ng/µL</li> |
| | + | <li>Calculation of volume needed for ligation: |
| | + | <li>c(mVenus_YFP) = 3,66 µL</li> |
| | + | <li>c(pCMV_MCS) = 5,34 µL</li></li> |
| | </ul> | | </ul> |
| - | <p style="font-size:13px; color:#b2222;"><b>Sequencing results of pSB1C3_CMV: </b></p>
| |
| - | <br />
| |
| - | <ul>
| |
| - | <li>Plasmid sent for sequencing: </li>
| |
| - | <li>Primer used: VR-2 </li>
| |
| - | <li><b>Results:</b> Sequence read looks good. The incorporation of the PCR product can be confirmed. It can be seen that the insert is in the RFC10 standard and prefix and suffix have the right sequence. Therefore, this BioBrick can be send to the registry and used for BioBrick assembly.But: there is a mutation in the termintor (sequence picture do not show this mutation)</li>
| |
| - | </ul>
| |
| - |
| |
| | | | |
| - | <img src="http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/images/7/74/Freiburg10_Sequence_analysis_of_PSB1C3_CMV.jpg" />
| + | <b>Transformation</b> |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#b2222;"><b>Sequencing results of pSB1C3_hGH: </b></p>
| + | |
| - | <br />
| + | |
| | <ul> | | <ul> |
| - | <li>Plasmid sent for sequencing: </li> | + | <li>Transformation has been followed the standard protocol [[Media:Freiburg10_Cloning Protocol.pdf]] </li> |
| - | <li>Primer used: VR-2 </li>
| + | |
| - | <li><b>Results:</b> Sequence read looks good. The incorporation of the PCR product can be confirmed. It can be seen that the insert is in the RFC10 standard and prefix and suffix have the right sequence. Therefore, this BioBrick can be send to the registry and used for BioBrick assembly.</li>
| + | |
| | </ul> | | </ul> |
| - | http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/images/e/e1/Freiburg10_Sequence_analysis_of_PSB1C3_betaglobin.jpg
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#b2222;"><b>Sequencing results of pSB1C3_hGH: </b></p>
| |
| - | <br />
| |
| - | <ul>
| |
| - | <li>Plasmid sent for sequencing: </li>
| |
| - | <li>Primer used: VR-2 </li>
| |
| - | <li><b>Results:</b> Sequence read looks good. The incorporation of the PCR product can be confirmed. It can be seen that the insert is in the RFC10 standard and prefix and suffix have the right sequence. Therefore, this BioBrick can be send to the registry and used for BioBrick assembly.</li>
| |
| - | </ul>
| |
| - | http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/images/4/44/Freiburg10_Sequence_analysis_of_PSB1C3_hGH.jpg
| |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b> Cellculture </b></p>==== | + | ===12. Labortag 20.05.2010=== |
| - | Investigator: Adrian<br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | The Motivation: Transfection<br />
| + | |
| - | The Action: HEK cells were split into four T75 flasks<br />
| + | |
| - | The Plan: The cells should be ready at saturday for seeding and monday for transfection<br />
| + | |
| - | <br /><br />
| + | |
| - | We recived new HT1080 cells, they'll be split tomorrow (thx to Sven)
| + | |
| - | <br /><br />
| + | |
| - | We recived new tumor cells which overexpress EGFR (thx to barbara)
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Picking clones of pSB1C3_AAP_2, pSB1C3_AAP, pSB1C3_Rep78_2, pSB1C3_Rep78, pSB1C3_Rep68_2 and pSB1C3_Rep68</b></p>==== | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Picking clones</b></p>==== |
| - | <br/>
| + | |
| - | Investigator: Anissa
| + | |
| - | <br/>
| + | |
| - | Of each construct two clones were picked. plates are still stored in the cold-room, tomorrow mini-prep will be done.
| + | |
| - | <br />
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>PCR and ligation for biobrick-production of pSB1C3_hTERT</b></p>====
| + | <p><b>Investigators: Adrian, Bea</b></p> |
| - | <br/> | + | <ul><br/> |
| - | Investigator: Anissa,Kerstin<br />
| + | Clones were picked according to the standard protocol. |
| - | <br />
| + | *3 approaches from each plate</li> |
| - | <br />
| + | </ul> |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': PCR was performed one time without DMSO and one time with DMSO. Only the approach with DMSO showed bands in the analytic gel after PCR. That's why only this approach will be noted. </p> <br>
| + | |
| - | <br>
| + | |
| - | *'''PCR: | + | |
| - | (was performed following the standard protocol)
| + | |
| - | <br> | + | |
| - | {| border="1"
| + | |
| - | | '''Ingredients''' || align="right" |'''Volume / µl'''
| + | |
| - | |-
| + | |
| - | | 5X Phusion HF buffer || align="right" |10
| + | |
| - | |-
| + | |
| - | | 10 mM dNTP mix|| align="right" |1
| + | |
| - | |-
| + | |
| - | | forward primer: O111 (phTERT_prefix_for_RFC10) || align="right" |2,5
| + | |
| - | |-
| + | |
| - | | reverse primer: O112 (phTERT_suffix_rev_RFC10) || align="right" |2,5
| + | |
| - | |-
| + | |
| - | | DNA Template|| align="right" |0,35 µl
| + | |
| - | |-
| + | |
| - | | DMSO (2%)|| align="right" | 1
| + | |
| - | |-
| + | |
| - | | Phusion Polymerase|| align="right" |0,5
| + | |
| - | |-
| + | |
| - | |H<sub>2</sub>O|| align="right" |32,15
| + | |
| - | |-
| + | |
| - | |Total volume|| align="right" |50
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| | | | |
| - | PCR program:
| + | ====13. Labortag 21.05.2010: CMV-Promoter==== |
| - | {| border="1"
| + | |
| - | |'''PCR Program'''|| align="right" |'''temperature/ °C'''
| + | |
| - | |-
| + | |
| - | |1|| align="right" |98
| + | |
| - | |-
| + | |
| - | |2|| align="right" |98
| + | |
| - | |-
| + | |
| - | |8x|| align="right" |58
| + | |
| - | |-
| + | |
| - | |3 || align="right" |72
| + | |
| - | |-
| + | |
| - | |4 || align="right" |98
| + | |
| - | |-
| + | |
| - | |17x|| align="right" |70
| + | |
| - | |-
| + | |
| - | |5|| align="right" |72
| + | |
| - | |-
| + | |
| - | |6x|| align="right" |72
| + | |
| - | |-
| + | |
| - | |Hold|| align="right" |4
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| - | | + | <b>Theoretical cloning:</b> (Volker, Hanna) |
| - | | + | * The CMV promoter (mainly the regulatory region) was further characterized: For this purpose U.S. patent no. 5,385,839 was used. [[Media:Freiburg10_Patent_US5385839A.pdf]] |
| - | <gallery widths=500px heights=300px perrow=2 caption="BioBrick production of pSB1C3_hTERT"> | + | * Further on the CMV promoter sequence was blasted. The results delivered a 98% query coverage with the "Human herpesvirus 5 strain Toledo, complete genome" (accession no.: GU937742.1). Interestingly the maximal identity was just 99%. This can be explained due to a nucleotide deletion and a C-T transition (red circles), which were also marked in Geneious. |
| - | Image:Freiburg10_pSB1C3 cut with XbaI and PstI for TERT promoter.jpg|Preparative gel
| + | [[File:Freiburg10_CMV-Alignment.jpg|500x500px|]] |
| - | Image:Freiburg10_hTERT after PCR.jpg|analytic gel
| + | |
| - | </gallery> | + | |
| - | <br/>
| + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Gel extraction</p>
| + | |
| - | <br />
| + | |
| - | Gel measurement:
| + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | '''Sample'''
| + | |
| - | | align="left" | '''Weight'''
| + | |
| - | | align="left" | '''Volume'''
| + | |
| - | | align="left" | '''Concentration'''
| + | |
| - | |-
| + | |
| - | | align="left" | hTERT
| + | |
| - | | align="left" | 0,15g
| + | |
| - | | align="left" | 20 µl
| + | |
| - | | align="left" | 37,49 ng/µl
| + | |
| - | |-
| + | |
| - | | align="left" | pSB1C3_cut | + | |
| - | | align="left" | 0,08g
| + | |
| - | | align="left" | 20 µl
| + | |
| - | | align="left" | 4,56 ng/µl
| + | |
| - | |} | + | |
| | <br> | | <br> |
| - | | + | [[File:Freiburg10 CMV.jpg|800x800px|]] |
| - | *Digestion of PCR product and vector:
| + | |
| - | | + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | '''components''' || align="right" |'''PCR product /µl''' || align="right" |'''vector /µl'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |18|| align="right" |7,58
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |0,3 (100X used)|| align="right" | 2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |2,5 || align="right" |2
| + | |
| - | |-
| + | |
| - | |Enzyme '''XbaI || align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |Enzyme '''PstI || align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |2,2 || align="right" |6,42
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 25|| align="right" |20
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | Ligation with T4-Ligase and transformation with BL 21 was made. Clones have to be picked tomorrow.
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Transformation evaluation of XL1B cells and repetition of transformation</b></p>====
| + | |
| - | | + | |
| - | Investigator: Kira <br />
| + | |
| - | Against our expectations both agar plates contain very few colonies. In order to figure out if the lack of colonies is due to the XL1 blue cells or loss of pUC activity, test transformation will be repeated this evening with recently prepared XL1 blue cells as well as with Lab-XL1B cells.
| + | |
| - | 50 pg pUC-plate contains 8 colonies
| + | |
| - | 100 pg pUC-plate contains 18 colonies
| + | |
| - | Transformation will be performed again with 100 pg pUC (= 2 ㎕ pUC).
| + | |
| - | <br />
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Cloning of SDM SspI and SDM PvuII'''</p>====
| + | |
| - | '''Investigator: Chris W.'''
| + | |
| - | *Vector: name: pSB1C3_SDM_SspI '''P125'''
| + | |
| - | *Insert: name: pSB1C3_SDM_PvuII '''P129'''
| + | |
| - | *new vector name: pSB1c3_SDM_SspI/PvuII '''P157'''
| + | |
| - | *buffer used: 4 ; Restriction-enzymes used: Enzyme 1 (no. Lab: 144) BpmI ; Enzyme 2 (no.Lab: 152) ClaI
| + | |
| - | *DNA concentration (vector): 321,9 ng/µl ; DNA concentration (insert): 308,4 µg/µl
| + | |
| - | | + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | '''components''' || align="right" |'''volume of pSB1C3_SDM_SspI /µl''' || align="right" |'''volume of pSB1C3_SDm_PvuII /µl'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |4,66 || align="right" |4,87
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |2 || align="right" | 2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2
| + | |
| - | |-
| + | |
| - | |Enzyme BpmI (no.Lab:144)|| align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |Enzyme ClaI (no.Lab:152)|| align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |9,34 || align="right" |9,13
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 20|| align="right" |20
| + | |
| - | |}
| + | |
| - | <br> <p style="color:red;">Comment: I guess enzyme 144 was BpmI and enzyme 152 was ClaI ? (Jessica)</p> <br>
| + | |
| - | <br> <p style="color:red;">Comment: u r right, changed (Chris)</p> <br>
| + | |
| - | <br />
| + | |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 130 Volt, running time:45
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | | + | |
| - | <br />
| + | |
| - | '''Loading plan for agarose gel''': <br>
| + | |
| - | Marker used: GeneRuler ladder mix (Fermentas)<br>
| + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker
| + | |
| - | !Sample 125, 20µl
| + | |
| - | !Sample 129, 20µl
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |1
| + | |
| - | |5
| + | |
| - | |7
| + | |
| - | |- | + | |
| - | |} | + | |
| | <br> | | <br> |
| - | [[File:Gel1.png|500px|left|thumb|]]
| + | '''To do''': find "+1"-location (transcription start); which transcription factors bind to the regulatory region of the CMV promoter? |
| | <br> | | <br> |
| | <br> | | <br> |
| | + | '''LB medium''' was prepared: (Patrick and Chris W.) |
| | + | * 10 g Bacto-Tryptone, 5 g Bacto-Yeast, 10 g NaCl were mixed in 500 mL milipore-H2O. |
| | + | * Volume was adjusted to 1 L with milipore-H2O. |
| | + | * 100 mL flasks were each filled with 50 mL medium. |
| | + | * 0.75 g agar was added to each flask. |
| | + | * LB was sterilized by autoclaving and is now stored at room temperature. |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br>
| + | <b>Plasmid Mini-Prep according to the standard protocol</b> |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | | + | |
| - | The mutual vector (now without SspI) and insert (now without PvuII) were cut out. The gelextraction was performed according to the standard protocol. DNA concentration of the extracts:
| + | |
| - | * P125 Sspl: 12,8 ng/µl
| + | |
| - | * P129 PvuII: 11,6 ng/µl
| + | |
| - | <br>
| + | |
| - | The Ligation was performed as following:
| + | |
| - | * Vector Volume: 2,81 µl
| + | |
| - | * Insert Volume: 5,19 µl
| + | |
| - | <br>
| + | |
| - | * 1µl T4-Ligase buffer (10x)
| + | |
| - | * 8µl (Vector + Insert) mix
| + | |
| - | * 1µl T4-Ligase
| + | |
| - | <br> Incubate for 30min
| + | |
| - | | + | |
| - | <br>
| + | |
| - | '''Transformation:''' performed according to the standard protocol (BL21). The cells were plated on a agar plate with chloramphenicol
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Miniprep of p158 and p159</b></p>====
| + | |
| - | | + | |
| - | <p style="font-size:20px; font-weight: bold; color: blue;"><u>Plasmid Mini-Prep</u></p>
| + | |
| - | Investigator: Bea & Volker<br>
| + | |
| - | For the tripple mutant of pAAV-RC a Quick-change reaction was carried out to remove the last restriction site (SalI) that is required for the Viral Brick standard. For this construct glycerol stocks and minipreps were carried out for two clones.<br>
| + | |
| - | | + | |
| - | <br />
| + | |
| - | <u>Glycerol Stocks</u>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''Plasmidname''' ||align="left"| pAAV_RC_1.2 SDM SalI
| + | |
| - | ||align="left"| pAAV_RC_1.2 SDM SalI
| + | |
| - | |-
| + | |
| - | | align="left" | '''Date''' ||align="left"| 05.08.2010 ||align="left"| 05.08.2010
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B126 ||align="left"| B127
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| p158 ||align="left"| p159
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | TO do: Test digestion and sequencing!!!
| + | |
| - | | + | |
| - | ===81.Labortag 06.08.2010===
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Cloning Rep40 & Rep52 into pSB1C3</b></p>====
| + | |
| - | Intention: get biobrick ready <br>
| + | |
| - | Investigator: Patrick <br>
| + | |
| - | PCR of pKEX-2XL.Rep 40 (P22) and pKEX-2XL.Rep 52 (P23) following gelrun (1%) and gelextraction. <br>
| + | |
| - | Used Primers:
| + | |
| - | * Praefix Rep40_52ex (O94)
| + | |
| - | * Suffix Rep 40_68ex (O96)
| + | |
| - | * Suffix Rep 52_78ex (O97)
| + | |
| - | <br>
| + | |
| - | PCR programm of Rep40:
| + | |
| - | *98°C 1 min
| + | |
| - | <br>
| + | |
| - | *98°C 15 sec
| + | |
| - | *63°C 25 sec
| + | |
| - | *72°C 15 sec Repeat this cycle 8 times.
| + | |
| - | <br>
| + | |
| - | *98°C 15 sec
| + | |
| - | *68°C 25 sec
| + | |
| - | *72°C 15 sec Repeat this cycle 17 times.
| + | |
| - | <br>
| + | |
| - | *72°C 5 min
| + | |
| - | *Hold 4°C
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | PCR programm of Rep52:
| + | |
| - | *98°C 1 min
| + | |
| - | <br>
| + | |
| - | *98°C 15 sec
| + | |
| - | *62°C 25 sec
| + | |
| - | *72°C 20 sec Repeat this cycle 8 times.
| + | |
| - | <br>
| + | |
| - | *98°C 15 sec
| + | |
| - | *66°C 25 sec
| + | |
| - | *72°C 20 sec Repeat this cycle 17 times.
| + | |
| - | <br>
| + | |
| - | *72°C 5 min
| + | |
| - | *Hold 4°C
| + | |
| - | | + | |
| - | <br>
| + | |
| - | {| border="1"
| + | |
| - | |'''ingredients'''|| align="right" |'''Rep 40'''|| align="right" |'''Rep40 + DMSO'''|| align="right" |'''Rep52'''|| align="right" |''' Rep52 + DMSO
| + | |
| - | |-
| + | |
| - | | 5x Phusion HF buffer|| align="right" |10 µl || align="right" |10 µl|| align="right" |10 µl|| align="right" |10 µl
| + | |
| - | |-
| + | |
| - | |10 mM dNTP mix|| align="right" |1 µl || align="right" |1 µl|| align="right" |1 µl|| align="right" |1 µl
| + | |
| - | |-
| + | |
| - | |for Primer O94 (1:10 dilution, 05 µM)|| align="right" |2,5 µl || align="right" |2,5 µl|| align="right" |2,5 µl|| align="right" |2,5 µl
| + | |
| - | |-
| + | |
| - | |rev Primer (1:10 dilution, 0,5 µM) || align="right" |2,5 µl O96|| align="right" |2,5 µl O96|| align="right" |2,5 µl O97|| align="right" |2,5 µl O97
| + | |
| - | |-
| + | |
| - | |DNA template || align="right" | 1 µl, 112,7 ng/µl|| align="right" |1 µl, 112,7 ng/µl|| align="right" |0,9 µl, 142,3 ng/µl|| align="right" |0,9 µl, 142,3 ng/µl
| + | |
| - | |-
| + | |
| - | |DMSO|| align="right" |0 µl|| align="right" |0,5 µl|| align="right" |0 µl|| align="right" |0,5 µl
| + | |
| - | |-
| + | |
| - | |Phusion Polymerase|| align="right" |0,5 µl|| align="right" |0,5 µl|| align="right" |0,5 µl|| align="right" |0,5 µl
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |32,5 µl|| align="right" |32 µl|| align="right" |32,6 µl|| align="right" |32,1 µl
| + | |
| - | |-
| + | |
| - | |Total volume|| align="right" |50 µl|| align="right" |50 µl|| align="right" |50 µl|| align="right" |50 µl
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | | + | |
| - | Ecpected size of Rep40: 940 bp, expected size of Rep52:1198 bp. There are two samples of Rep40 and Rep52 and the PCR of one of each was run with 1% DMSO because the Rep40 Praefix has a strong secondary structure and was used as a primer for Rep 40 and Rep 52<br>
| + | |
| - | <br>
| + | |
| - | [[File:IM000026_Patrick.JPG|400px]]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | Digestion of pSB1C3 (P51.1) with EcoRI-HF and SpeI following gelrun (0.8%) and gelextraction. <br>
| + | |
| - | Expected size of the fragments: about 2000 bp and 800bp <br>
| + | |
| - | <br>
| + | |
| - | [[File:IM000028_Patrick.JPG|500px]]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | All marked fragments were extracted. The Gelextraction was performed according to the standard protocol following a digestion of the PCR products with EcoRI HF and SpeI and a purification of the PCR product. The Ligation was not performed according to the standard protocol: 1 µl T4 DNA Ligase, 1 µl (10x) Buffer, 3 µl vector pSB1C3 (60 ng/µl) and 5 µl insert (all concentrations about 100 ng/µl). The Transformation (with BL21) was performed according to the standard protocol. Tomorrow there will hopefully be some clones on the agar plates.
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>BioBrick Assembly of pSB1C3_beta globin_mVenus</b></p>====
| |
| - | Investigator: <b>Bea </b>
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: BioBricks are ready to use. The sequenced pSB1C3_beta globin (P133) and pGA14_mVenus (P60) will be digested with different enzymes and will be assembled in order to produce the first step in assembling the whole vector together. </p>
| |
| - | <br />
| |
| | <ul> | | <ul> |
| - | <li>First the plasmids P133 and P60 were digested:</li> | + | <li>investigator: Adrian, Kira, Anna, Chris W., Patrick</li> |
| - | </ul>
| + | <li>Measure DNA-concentration with Nanodrop</li> |
| - | <table border=2 cellpadding=1 cellspacing=1 width=528 style='border-collapse:
| + | |
| - | collapse;table-layout:fixed;width:396pt'>
| + | |
| - | <tr height=20 style='height:15.0pt'>
| + | |
| - | <td height=20 class=xl65 width=176 style='height:15.0pt;width:132pt'> </td>
| + | |
| - | <td class=xl66 width=176 style='border-left:none;width:132pt'>pSB1C3_betaglobin/µL</td>
| + | |
| - | <td class=xl66 width=176 style='border-left:none;width:132pt'>pGA14_mVenus/µL</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=20 style='height:15.0pt'>
| + | |
| - | <td height=20 class=xl67 width=176 style='height:15.0pt;border-top:none;
| + | |
| - | width:132pt'>DNA</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>7</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>9</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=20 style='height:15.0pt'>
| + | |
| - | <td height=20 class=xl67 width=176 style='height:15.0pt;border-top:none;
| + | |
| - | width:132pt'>BSA (100x)</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>0,2</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>0,2</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=40 style='height:30.0pt'>
| + | |
| - | <td height=40 class=xl67 width=176 style='height:30.0pt;border-top:none;
| + | |
| - | width:132pt'>Buffer 4<span style='mso-spacerun:yes'> </span>(10x)</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>2</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>2</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=40 style='height:30.0pt'>
| + | |
| - | <td height=40 class=xl67 width=176 style='height:30.0pt;border-top:none;
| + | |
| - | width:132pt'>Enzyme XbaI<span style='mso-spacerun:yes'> </span></td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>1</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>1</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=40 style='height:30.0pt'>
| + | |
| - | <td height=40 class=xl67 width=176 style='height:30.0pt;border-top:none;
| + | |
| - | width:132pt'>Enzyme AgeI</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>1</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>1</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=20 style='height:15.0pt'>
| + | |
| - | <td height=20 class=xl67 width=176 style='height:15.0pt;border-top:none;
| + | |
| - | width:132pt'>H2O</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>8,8</td>
| + | |
| - | <td class=xl68 style='border-top:none;border-left:none'>6,8</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=40 style='height:30.0pt'>
| + | |
| - | <td height=40 class=xl67 width=176 style='height:30.0pt;border-top:none;
| + | |
| - | width:132pt'>Total volume</td>
| + | |
| - | <td class=xl69 style='border-top:none;border-left:none'>20</td>
| + | |
| - | <td class=xl69 style='border-top:none;border-left:none'>20</td>
| + | |
| - | </tr>
| + | |
| - | <tr height=0 style='display:none'>
| + | |
| - | <td width=176 style='width:132pt'></td>
| + | |
| - | <td width=176 style='width:132pt'></td>
| + | |
| - | <td width=176 style='width:132pt'></td>
| + | |
| - | </tr>
| + | |
| - | </table>
| + | |
| - | <ul>
| + | |
| - | <li> Incubation of plasmids at 37°C for 2 hours </li>
| + | |
| - | <li> Load samples on preparative 1% agarose gel and run at 110 V, 45 minutes </li> | + | |
| | </ul> | | </ul> |
| - | <br/>
| |
| - | <b>Results:</b> As it can be seen in the picture below, digestion of the vector pSB1C3_betaglobin (left lane) revealed a band at around 2500 - 3000 bp. The expected size after digetsing with SpeI and PstI was: 2555 bp. The band ran a little bit higher than expected, anyhow the band was cut out of the gel. The right lane belongs to the pGA14_mVenus which was digested with XbaI and PstI which resulted in two fragments. mVENUS was digested. Expected sizes were: mVenus = 756bp and pGA14 = 2900bp. The expected sizes correspond to the bands seen in the picture below. The smaller fragment belonging to mVenus was cut out of the gel as well.
| |
| - | <br />
| |
| - | <br />
| |
| - | [[File:Freiburg10 pSB1C3 betaglobin mVenus 06 08 2010.jpg|550px]]
| |
| - | <br />
| |
| - | <br />
| |
| - | After gel extraction has been performed, the concentrations of the two fragments were measured and we obtained following concentrations:
| |
| - | <ul>
| |
| - | <li>c(pSB1C3_betaglobin) = 15,62 ng/µL </li>
| |
| - | <li>c(mVenus) = 6,83 ng/µL </li>
| |
| - | </ul>
| |
| - | <br />
| |
| - | For ligation the T4-Ligase were used.
| |
| - | <ul>
| |
| - | <li>v(pSB1C3_betaglobin) = 2,64 µL </li>
| |
| - | <li>v(mVenus) =5,36 µL </li>
| |
| - | <li>v(10xT4 Ligase buffer) = 1 µL </li>
| |
| - | <li>v(T4-Ligase) = 1 µL </li>
| |
| - | <li><b>Total volume = 10 µL </b></li>
| |
| - | </ul>
| |
| - | <br />
| |
| - | Additionally a control ligation was performed.
| |
| - | <ul>
| |
| - | <li>v(pSB1C3_betaglobin) = 2,64 µL </li>
| |
| - | <li>v(H<sub>2</sub>0) =5,36 µL </li>
| |
| - | <li>v(10xT4 Ligase buffer) = 1 µL </li>
| |
| - | <li>v(T4-Ligase) = 1 µL </li>
| |
| - | <li><b>Total volume = 10 µL </b></li>
| |
| - | <li>Ligation duration was 45 minutes on room temperature.</li>
| |
| - | </ul>
| |
| - | <br />
| |
| - | After ligation a transformation was performed.
| |
| - | <ul>
| |
| - | <li> Used bacterial strain: XL1-B</li>
| |
| - | <li> LB-agar plates containing chloramphenicol were plated and put in the 37°C room over night</li>
| |
| - | </ul>
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#003399;"><b>To do</b>: Mini-Prep and test digestion in order to verify assembly of beta globin and YFP. After verification of the fusion of the two fragments this construct is ready for the next BioBrick assembly step. </p>
| |
| | | | |
| - | ====<p style="font-size:15px; background-color:#ff00ff;">'''Test digestion of SalI SDM'''</p>====
| |
| - | Investigator: <b>Hanna</b>
| |
| - | <br/>
| |
| - | <p style="font-size:15px; font-weight: bold; color:#ff00ff;">Test digestion</p>
| |
| - | <ul>
| |
| - | <li>buffer used: 3 ; Restriction-enzymes used: Enzyme 1 (no. Lab: 53): SalI ; Enzyme 2: XbaI </li>
| |
| - | <li>Plasmid
| |
| - | <ul>
| |
| - | <li>Given Plasmid-Number: P158; DNA concentration: 398.8 ng/µL ;</li>
| |
| - | <li>Given Plasmid-Number: P159; DNA concentration: 437.4 ng/µL ;</li>
| |
| - | </ul>
| |
| - | </ul>
| |
| - | <br />
| |
| - | <br />
| |
| | {| border="1" | | {| border="1" |
| - | | align="left" | '''Components''' ||align="left"| '''P158 Volume/µL''' ||align="left"| '''P159 Volume/µL''' | + | | || align="right" | P3 (pCMV_mVenus_YFP) ||align="right"| P4 (pCMV_mVenus_YFP) ||align="right"| P5 (pCMV_mVenus_YFP) |
| - | |-
| + | |
| - | | align="left" | DNA ||align="left"| 2.5 ||align="left"| 2.3
| + | |
| - | |-
| + | |
| - | | align="left" | BSA (10x) ||align="left"| 1 ||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | Buffer 3 (10x) ||align="left"| 1 ||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | SalI (no. Lab: 53) ||align="left"| 0.5 ||align="left"| 0.5
| + | |
| - | |-
| + | |
| - | | align="left" | XbaI ||align="left"| 0.75 ||align="left"| 0.75
| + | |
| - | |-
| + | |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 4.25 ||align="left"| 4.45
| + | |
| - | |-
| + | |
| - | | align="left" | <b>Total volume</b> ||align="left"| <b>10</b> ||align="left"| <b>10</b>
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | Incubation: 1 h
| + | |
| - | <br />
| + | |
| - | <p style="font-size:15px; font-weight: bold; color:#ff00ff;">Agarose-Gel:</p>
| + | |
| - | <br />
| + | |
| - | 0.5 g Agarose, 50 mL TBE (1 %),3 µL GELRED, at 115 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample/µl]
| + | |
| - | !Loading dye (5x)/µl
| + | |
| - | !Expected size 1 (if SDM didn't work)
| + | |
| - | !Expected size 2 (if SDM didn't work)
| + | |
| - | !Expected size 3 (if SDM didn't work)
| + | |
| - | |--
| + | |
| - | |P158
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |6129 bp
| + | |
| - | |1143 bp
| + | |
| - | |61 bp
| + | |
| - | |--
| + | |
| - | |P159
| + | |
| - | |10 µl
| + | |
| - | |2 µl
| + | |
| - | |6129 bp
| + | |
| - | |1143 bp
| + | |
| - | |61 bp
| + | |
| - | |--
| + | |
| - | |}
| + | |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker /µL
| + | |
| - | !Sample P158 /µl
| + | |
| - | !Sample P159 /µl
| + | |
| - | !Marker /µL
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |6.5
| + | |
| - | |10
| + | |
| - | |10
| + | |
| - | |7
| + | |
| | |- | | |- |
| | + | | concentration (ng/µl)|| align="right" | 457||align="right"|470,8|| align="right" | 477,29 |
| | |} | | |} |
| - | <br />
| |
| - | [[File:Freiburg10 Test digestion SalI1.jpg|600px]] <br/>
| |
| | | | |
| - | <b>Comments:</b> Test digestion looked good: No 1143 bp fragment was detectable in neither test digestion. <br/>
| + | ===14. Labortag 25.05.2010=== |
| - | P158 was sent for sequencing to GATC. <br/>
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#ff00ff;">'''ITR test digestion of pAAV_MCS'''</p>==== | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Cloning of pCMV_mVenus_YFP + pAAV_MCS</b></p>==== |
| - | Investigator: <b>Hanna</b>
| + | |
| - | <br/>
| + | |
| - | <p style="color:#ff00ff;"><b>Comment:</b> Because sequencing of the right ITR delivered that perhaps a 15 bp fragment is missing in the sequence, we wanted to check, whether this fragment is also not present in the original pAAV_MCS plasmid or whether the loss of these 15 bp happened during cloning. </p>
| + | |
| - | <br/>
| + | |
| - | <p style="font-size:15px; font-weight: bold; color:#ff00ff;">Test digestion</p>
| + | |
| - | <ul>
| + | |
| - | <li>buffer used: 4 ; Restriction-enzymes used: Enzyme 1: NotI; Enzyme 2: PstI</li>
| + | |
| - | <li>Plasmid
| + | |
| - | <ul>
| + | |
| - | <li>Plasmid-Number: from Sven; DNA concentration: 259 ng/µL ;</li>
| + | |
| - | </ul>
| + | |
| - | </ul>
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | '''Components''' ||align="left"| '''Volume/µL'''
| + | |
| - | |-
| + | |
| - | | align="left" | DNA ||align="left"| 3.8
| + | |
| - | |-
| + | |
| - | | align="left" | BSA (10x) ||align="left"| 1.5
| + | |
| - | |-
| + | |
| - | | align="left" | Buffer 4 (10x) ||align="left"| 1.5
| + | |
| - | |-
| + | |
| - | | align="left" | NotI-HF ||align="left"| 0.75
| + | |
| - | |-
| + | |
| - | | align="left" | PstI-HF ||align="left"| 0.75
| + | |
| - | |-
| + | |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 3.2
| + | |
| - | |-
| + | |
| - | | align="left" | <b>Total volume</b> ||align="left"| <b>15</b>
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | Incubation: 1 h
| + | |
| - | <br />
| + | |
| - | <p style="font-size:15px; font-weight: bold; color:#ff00ff;">Agarose-Gel:</p>
| + | |
| - | <br />
| + | |
| - | 0.5 g Agarose, 50 mL TBE (1 %),3 µL GELRED, at 115 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample/µl]
| + | |
| - | !Loading dye (5x)/µl
| + | |
| - | !Expected size <b>ITRs</b>
| + | |
| - | !Expected size 2
| + | |
| - | !Expected size 3
| + | |
| - | !Expected size 4
| + | |
| - | |--
| + | |
| - | |pAAV_MCS
| + | |
| - | |15 µl
| + | |
| - | |2 µl
| + | |
| - | |145 bp
| + | |
| - | |1216 bp
| + | |
| - | |555 bp
| + | |
| - | |2608 bp
| + | |
| - | |--
| + | |
| - | |}
| + | |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker /µL
| + | |
| - | !Sample /µl
| + | |
| - | !Marker /µL
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |6.5
| + | |
| - | |12
| + | |
| - | |6.5
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | [[File:Freiburg10 testDigestion pAAV MCS.jpg|700px]] <br/>
| + | |
| | | | |
| - | <p style="font-size:15px; color:#ff00ff;"><b>Comments:</b> The test digestion showed that there're two bands between the 100 and 200 bp marker nucleotides! By comparing the gel picture with the last mini-prep digestion of the fancy method we found out that the space between the two bands are similar. Because of that we assumed that the 15 pb are already missing in the original Stratagene plasmid!!! </p> <br/> | + | <p><b>Investigators: Kira, Anna, Volker, Jessica</b></p> |
| - | <p style="font-size:15px; color:#800080;"><b>Conclusion:</b> Because we already tested the Stratagene Kit via YFP expression, we can conclude that the usage of the "wrong" rigth ITR works. Therefore we decided to continue working with this "mutated" = <b> ENGINEERED </b> :) right ITR as BioBrick.</p> <br/>
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Miniprep of pSBC13_Rep78, pSB1C3_Rep68, pSB1C3_AAP, pSB1C3_rightITR and psB1C3_leftITR</b></p>====
| |
| - |
| |
| - | <p style="font-size:13px; color: blue;">Plasmid Mini-Prep</p>
| |
| - | Investigator: Kerstin<br>
| |
| | <br> | | <br> |
| | | | |
| - | <br /> | + | <li>plasmid: insert: pCMV_mVenus_YFP; number: P5 production date: 21.05.2010 origin: ____ |
| - | <u><b>Glycerol Stocks</b></u> <br>
| + | <li>plasmid: vector: pAAV_MCS; number: P? production date: ____ origin: ____ |
| - | <b>pSB1C3_Rep78</b>
| + | <li>new vector name: pAAV_mVenus_YFP <br> |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B128 ||align="left"| B129
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P160 ||align="left"| p161
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 139,1 ||align="left"| 71,0
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_2_Rep78</b>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B130 ||align="left"| B131
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P162 ||align="left"| p163
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 112,1 ||align="left"| 79,7
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_Rep68</b> | + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B132 ||align="left"| B133
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P164 ||align="left"| p165
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 80,2 ||align="left"| 72,8
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_2_Rep68</b> | + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B134 ||align="left"| B135
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P166 ||align="left"| p167
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 85,6 ||align="left"| 69,1
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_AAP</b>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B136 ||align="left"| B137
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P168 ||align="left"| p169
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 63,6 ||align="left"| 56,3
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_2_AAP</b>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B138 ||align="left"| B139
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P170 ||align="left"| p171
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 135,1 ||align="left"| 114,2
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_rightITR</b>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B140 ||align="left"| B141
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P172 ||align="left"| p173
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 73,7 ||align="left"| 66,4
| + | |
| - | |}
| + | |
| - | <b>pSB1C3_leftITR</b>
| + | |
| - | {| border="1"
| + | |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2'''
| + | |
| - | |-
| + | |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL-21 ||align="left"| BL-21
| + | |
| - | |-
| + | |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B142 ||align="left"| B143
| + | |
| - | |-
| + | |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P174 ||align="left"| p175
| + | |
| - | |-
| + | |
| - | | align="left" | '''DNA-concentration ng/µl''' ||align="left"| 74,9 ||align="left"| 80,3
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| - | '''Test digestion:'''<br>
| + | |
| - | pSB1C3_Rep78, pSB1C3_Rep68 and pSB1C3_AAP were digested with XbaI and SpeI<br>
| + | |
| - | pSB1C3_rightITR and pSB1C3_leftITR were digested with EcoRI and PstI.<br>
| + | |
| | | | |
| | <br> | | <br> |
| - | {| border="1"
| + | '''1st try: |
| - | | components || align="right" |volume of '''P160'''/µl ||volume of '''P161'''/µl||volume of '''P162/'''µl ||volume of '''P163'''/µl ||volume of '''P164'''/µl||volume of '''P165'''/µl||volume of '''P166'''µl ||volume of '''P167'''/µl||volume of '''P168'''µl ||volume of '''P169'''/µl||volume of '''P170'''/µl||volume of '''P171'''/µl||volume of '''P172'''/µl||volume of '''P173'''µl ||volume of '''P174'''/µl ||volume of '''P175'''/µl
| + | |
| - | |-
| + | |
| - | | DNA ||5,8 ||11,3 ||7,1 ||10 ||9,9 ||10,9 ||9,3 ||11,6 ||12,5 ||14,2 ||5,9 ||7,0 ||10,9 ||12,0 ||10,7 ||9,7
| + | |
| - | |-
| + | |
| - | | BSA (10x) ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2 ||2
| + | |
| - | |-
| + | |
| - | |Enzyme 1 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5
| + | |
| - | |-
| + | |
| - | |Enzyme 2 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5 ||0,5
| + | |
| - | |-
| + | |
| - | |H2O ||9,2 ||3,7 ||7,9 ||5,0 ||5,1 ||4,1 ||5,7 ||3,4 ||2,5 ||0,8 ||9,1 ||8 ||4,1 ||3 ||4,3 ||5,3
| + | |
| - | |-
| + | |
| - | |'''Total volume /µl'''||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20 ||20
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | '''Expected sizes:'''<br>
| + | |
| - | *Rep78: 1,8 kb
| + | |
| - | *Rep68: 1,6kb<br>
| + | |
| - | *AAP: 650bp <br>
| + | |
| | | | |
| | + | <li>buffer used:3 ; Restriction-enzymes used: Enzyme NotI (no. Lab:46) |
| | + | <li>DNA concentration (vector): 360 ng/µl ; DNA concentration (insert): 477 ng/µl |
| | | | |
| - | *left ITR: 150bp
| |
| - | *right ITR: 150bp
| |
| - |
| |
| - | <br>
| |
| - | <p style="font-size:13px; color:#11bb88;">'''Results of samples loaded 1,5% agarose gel and ran ~50 minutes at 115 V:''' </p>
| |
| - | [[File:Freiburg10 test digestion itr und aap.jpg|400px]]
| |
| - | <br>
| |
| - | <p style="font-size:13px; color:#11bb88;">'''Results of samples loaded 0,8% agarose gel and ran ~50 minutes at 115 V:''' </p>
| |
| - | [[File:Freiburg10 test digestion rep 78 u. 68.jpg|400px]]
| |
| - |
| |
| - |
| |
| - |
| |
| - | <p style="font-size:13px; color:#11bb56;">'''Sequencing''' </p>
| |
| - | P160, P165 and P170 were send for sequencing.
| |
| - | ===82.Labortag 07.08.2010===
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Cellculture</b></p>====
| |
| - |
| |
| - | HEK 293 cells were split and seeded according to the standard protocol: 2x T75 flask and 10x10cm cellculture dishes. Investigator: Patrick
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Continuation: Cloning Rep40 & Rep52 into pSB1C3</b></p>====
| |
| - | Investigator: Patrick <br>
| |
| - | Results: no clones could be picked because there were no clones. Maybe the PCR purification or one of the following steps did not work (the PCR purifications were quite bad) so i purified the PCR products again yielding very low DNA concentrations (0-13,28 ng/µl) and still very obvious pollution of the sample. Nonetheless a ligation with the insert Rep40+DMSO, Rep52, Rep52+DMSO and the vector pSB1C3 was carried out following a transformation and according to the standard protocols. Hopefully there will be some clones tomorrow.
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep and test digestion of pSB1C3_SDM_SspI/PvuII</b></p>====
| |
| - | '''Investigator: Jessica'''<br>
| |
| - | <br>
| |
| - | <p style="font-size:18px; font-weight: bold; color:#66bbff;"><u>Plasmid Mini-Prep</u></p>
| |
| - | *new vector name: pSB1C3_SDM_SspI/PvuII
| |
| - | <br />
| |
| - | <u><b>Glycerol Stocks</b></u> <br>
| |
| - | <b>pSB1C3_SDM_SspI/PvuII</b>
| |
| | {| border="1" | | {| border="1" |
| - | | align="left" | ||align="left"| '''Clone 1''' ||align="left"| '''Clone 2''' ||align="left"| '''Clone 3''' | + | | components || align="right" | V (pCMV_mVenus_YFP)/ µl ||align="right"| V(pAAV_MCS) / µl |
| | |- | | |- |
| - | | align="left" | '''Bacteria strain''' ||align="left"| BL21 ||align="left"| BL21 ||align="left"| BL21 | + | | DNA || align="right" | 4.2||align="right"|3.5 |
| | |- | | |- |
| - | | align="left" | '''Plasmidname''' ||align="left"| pSb1C3_SDM_SspI/PvuII ||align="left"| pSb1C3_SDM_SspI/PvuII ||align="left"| pSb1C3_SDM_SspI/PvuII | + | | BSA (10x) || align="right" |3||align="right"|3 |
| | |- | | |- |
| - | | align="left" | '''Date''' ||align="left"| 7.8.10 ||align="left"| 7.8.10 ||align="left"| 7.8.10 | + | | Buffer 3 (10x)|| align="right" |3||align="right"|3 |
| | |- | | |- |
| - | | align="left" | '''given glycerol-stock no.''' ||align="left"| B144 ||align="left"| B145 ||align="left"| B146 | + | |Enzyme: NotI (no.Lab:46)|| align="right" |1||align="right"|1 |
| | |- | | |- |
| - | | align="left" | '''given plasmid no.''' ||align="left"| P157.1 ||align="left"| P157.2 ||align="left"| P157.3 | + | |H2O|| align="right" |15.3||align="right"|15.3 |
| - | |} | + | |
| - | <br>
| + | |
| - | | + | |
| - | | + | |
| - | <p style="font-size:15px; font-weight: bold; color:#66bbff;">Test digestion</p>
| + | |
| - | *buffer used: 2 ; Restriction-enzymes used: Enzyme 1 SspI ; Enzyme 2 PvuII
| + | |
| - | *Plasmid: pSB1C3_SDM_SspI/PvuII:
| + | |
| - | **Given Plasmid-Number: P157.1; DNA concentration: 246,3 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P157.2; DNA concentration: 231,7 ng/µL ;
| + | |
| - | **Given Plasmid-Number: P157.3; DNA concentration: 219,6 ng/µL ;
| + | |
| - | <br />
| + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | | align="left" | '''Components''' ||align="left"| <b>P157.1</b> Volume/µL ||align="left"| <b>P157.2</b> Volume/µL ||align="left"| <b>P157.3</b> Volume/µL | + | |
| - | |-
| + | |
| - | | align="left" | DNA ||align="left"| 2,8 ||align="left"| 3,0 ||align="left"| 3,19
| + | |
| - | |-
| + | |
| - | | align="left" | BSA (10x) ||align="left"| - ||align="left"| - ||align="left"| -
| + | |
| - | |-
| + | |
| - | | align="left" | Buffer no. 2 (10x) ||align="left"| 1 ||align="left"| 1 ||align="left"| 1
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 1 SspI ||align="left"| 0.5 ||align="left"| 0.5 ||align="left"| 0.5
| + | |
| - | |-
| + | |
| - | | align="left" | Enzyme 2 PvuII ||align="left"| 0.5 ||align="left"| 0.5 ||align="left"| 0.5
| + | |
| - | |-
| + | |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 5,2 ||align="left"| 5 ||align="left"| 4,81
| + | |
| | |- | | |- |
| - | | align="left" | '''Total volume''' ||align="left"| <b>10</b> ||align="left"| <b>10</b> ||align="left"| <b>10</b>
| + | |'''Total volume'''|| align="right" |<b>26.4</b>||align="right"|<b>25.8</b> |
| | |} | | |} |
| - | <br />
| |
| | | | |
| - | *Incubation: 45 min
| + | <li> Incubation: 1 1/2 h at 37°C |
| - | <br /> | + | |
| - | <p style="font-size:15px; font-weight: bold; color:#66bbff;">Agarose-Gel:</p>
| + | |
| - | <br />
| + | |
| - | 0.45 g Agarose, 50 mL <b>TAE</b>, 3 µL GELRED, at 115 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample/µl]
| + | |
| - | !Loading dye (6x)/µl
| + | |
| - | | + | |
| - | |--
| + | |
| - | |P157.1
| + | |
| - | |10 µl
| + | |
| - | |1,6 µl
| + | |
| - | | + | |
| - | |--
| + | |
| - | |P157.2
| + | |
| - | |10 µl
| + | |
| - | |1,6 µl
| + | |
| - | | + | |
| - | |--
| + | |
| - | |P157.3
| + | |
| - | |10 µl
| + | |
| - | |1,6 µl
| + | |
| - | | + | |
| - | |--
| + | |
| - | | + | |
| - | |}
| + | |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker /µL
| + | |
| - | !Sample P157.1 /µL
| + | |
| - | !Sample P157.2 /µL
| + | |
| - | !Sample P157.3 /µL
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |7
| + | |
| - | |10
| + | |
| - | |10
| + | |
| - | |10
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| | <br> | | <br> |
| - | I expect one cut from the PvuII RS in the CFP --> linearized <br>
| + | '''note: too little water was added |
| - | [[File:Prep pSB1C3_SDM_SspIPvuII.jpg|400px]] <br>
| + | |
| | <br> | | <br> |
| - | <b>Comment:</b><br>
| |
| | <br> | | <br> |
| - | <p style="font-size:13px; color:#ff0000;">'''''Comments:'''''Oh f... it makes no sense... if the cloning didn't work, there can just be 3 bands (one time SspI and twice PvuII). The results of the test digestion from aug 1th showed that there can be one RS PvuII more. but then you have a maximum of 4 bands. '''P157.2''' show '''5''' bands. i can't interpret this result at the moment. interpretation will follow</p> <br>
| + | 1% Agarose gel |
| - | <br/>
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Evaluation of test transformation with XL1 blue cells and pUCI</b></p>====
| + | |
| - | '''Investigator: Kira'''<br>
| + | |
| - | | + | |
| - | Transformation was performed with lab XL1B and iGEM XL1B cells. iGEM plate contained just 25 colonies, while lab plate contained around 50 colonies.
| + | |
| - | Conclusion: the production of competent cells has to be repeated.
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Biobrick production of p5 promoter from pTAV2 and pAAV_RC</b></p>====
| + | |
| - | '''Investigator: Kira'''<br>
| + | |
| - | *'''PCR:
| + | |
| - | | + | |
| - | DNA samples were diluted 1:100
| + | |
| - | | + | |
| | <br> | | <br> |
| - | {| border="1"
| + | <li>1% agarose gel was prepared, gel ran for 45 minutes(119 V) |
| - | | '''Ingredients''' || align="right" |'''p 32''' ||align="right"|p 50
| + | |
| - | |-
| + | |
| - | | 5X Phusion HF buffer || align="right" |10 µl||align="right"|10 µl
| + | |
| - | |-
| + | |
| - | | 10 mM dNTP mix|| align="right" |1µl ||align="right"|1 µl
| + | |
| - | |-
| + | |
| - | | forward primer: O114 || align="right" |2,5µl||align="right"|2,5 µl
| + | |
| - | |-
| + | |
| - | | reverse primer: O115 || align="right" |2,5 µl||align="right"|2,5 µl
| + | |
| - | |-
| + | |
| - | | DNA Template|| align="right" |0,3 µl||align="right"| 0,4 µl
| + | |
| - | |-
| + | |
| - | | DMSO || align="right" |0 µl||align="right"|0 µl
| + | |
| - | |-
| + | |
| - | | Phusion Polymerase|| align="right" |0,5 µl||align="right"| 0,5 µl
| + | |
| - | |-
| + | |
| - | |H<sub>2</sub>O|| align="right" |33,2 µl||align="right"|28,6 µl
| + | |
| - | |-
| + | |
| - | |Total volume|| align="right" |50 µl||align="right"| 50 µl
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| - |
| |
| - | PCR program:
| |
| - |
| |
| - | {| border="1"
| |
| - | |Cycles||Temperature||Time
| |
| - | |-
| |
| - | |||98°C||1
| |
| - | |-
| |
| - | |8x||98°C||30"
| |
| - | |-
| |
| - | |||60°C||25"
| |
| - | |-
| |
| - | |||72°C||6"
| |
| - | |-
| |
| - | |17x||98°C||30"
| |
| - | |-
| |
| - | |||70°C||25"
| |
| - | |-
| |
| - | |||72°C||6"
| |
| - | |-
| |
| - | |1x||72°C||5'
| |
| - | |-
| |
| - | |Hold 4°C
| |
| - | |}
| |
| | <br> | | <br> |
| | + | '''2nd try:''' |
| | | | |
| - | Digestion of plasmid backbone:
| + | <li>buffer used:4 ; Restriction-enzymes used: Enzyme NotI (no. Lab:159) |
| - | | + | <li>DNA concentration (vector): 360 ng/µl ; DNA concentration (insert): 477 ng/µl |
| - | c (pSB1C3) = 151, 1 ng/ µl
| + | |
| | | | |
| | {| border="1" | | {| border="1" |
| - | | align="left" | '''Components''' ||align="left"| <b>vector</b> Volume/µL | + | | components || align="right" | V (pCMV_mVenus_YFP)/ µl ||align="right"| V(pAAV_MCS) / µl |
| | |- | | |- |
| - | | align="left" | DNA 1 µg ||align="left"| 6,0 µl | + | | DNA || align="right" | 4.2||align="right"|3.5 |
| | |- | | |- |
| - | | align="left" | BSA (100x) ||align="left"| 0,2 µl | + | | BSA (10x) || align="right" |3||align="right"|3 |
| | |- | | |- |
| - | | align="left" | Buffer no. 4 (10x) ||align="left"| 2,0 µl
| + | | Buffer 4 (10x)|| align="right" |3||align="right"|3 |
| | |- | | |- |
| - | | align="left" | Enzyme 1 XbaI ||align="left"| 1 µl | + | |Enzyme: NotI (no.Lab:159)|| align="right" |1,5||align="right"|1,5 |
| | |- | | |- |
| - | | align="left" | Enzyme 2 PstI HF ||align="left"| 1 µl | + | |H2O|| align="right" |18,3||align="right"|19 |
| | |- | | |- |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 9,8 µl | + | |'''Total volume'''|| align="right" |<b>30</b>||align="right"|<b>30</b> |
| - | |-
| + | |
| - | | align="left" | '''Total volume''' ||align="left"| <b>20</b>
| + | |
| | |} | | |} |
| - | <br />
| |
| | | | |
| - | incubation @ 37 C for approx. 2 h
| + | <li> Incubation: 1 1/2 h at 37°C |
| | | | |
| - | 1% agarose gel with PCR products:
| |
| - |
| |
| - | [[File:Freiburg10 PCR.jpg|400px]]
| |
| - | <br />
| |
| - |
| |
| - | Concentrations after gel extraction:
| |
| - |
| |
| - | c(p32) = 67,95 ng/µl <br />
| |
| - | c(p50) = 32,45 ng/µl <br />
| |
| - | c(vector) = 7,54 ng/µl <br />
| |
| - | <br />
| |
| - |
| |
| - | Digestion of PCR products:
| |
| - |
| |
| - | {| border="1"
| |
| - | | align="left" | '''Components''' ||align="left"| <b>PCR product</b> Volume/µL
| |
| - | |-
| |
| - | | align="left" | DNA 1 µg ||align="left"| 23,0 µl
| |
| - | |-
| |
| - | | align="left" | BSA (100x) ||align="left"| 0,3 µl
| |
| - | |-
| |
| - | | align="left" | Buffer no. 4 (10x) ||align="left"| 3,0 µl
| |
| - | |-
| |
| - | | align="left" | Enzyme 1 XbaI ||align="left"| 1,5 µl
| |
| - | |-
| |
| - | | align="left" | Enzyme 2 PstI HF ||align="left"| 1,5 µl
| |
| - | |-
| |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 0,7 µl
| |
| - | |-
| |
| - | | align="left" | '''Total volume''' ||align="left"| <b>30</b>
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | Concentrations after PCR purification:
| |
| - |
| |
| - | c(p32) = 21, 63 ng/µl <br />
| |
| - | c(p50) = 19,48 ng/µl <br />
| |
| - | <br />
| |
| - |
| |
| - | Ligation:
| |
| - |
| |
| - | Quickligase was used for ligation.
| |
| - |
| |
| - | p32 DNA-Mix: 8 µl vector + 1 µl insert <br />
| |
| - | p50 DNA-Mix: 7,9 µl vector + 1,1 µl insert <br />
| |
| - | <br />
| |
| - | Transformation:
| |
| - |
| |
| - | Transformation was performed according to the standard protocol with DYT and BL21 cells. The cells were spread on the agar plates containing chloramphenicol.
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#ff00ff;">'''Sequencing results of SalI SDM'''</p>====
| |
| - | Investigator: <b>Hanna</b>
| |
| - | <br/>
| |
| - |
| |
| - | <p style="color:#ff00ff;"><b>Comment:</b> The test digestion already showed that the SDM of the SalI restriction site in pAAV_RC worked. This could be validated by sequencing the plasmid with the Rep-1250_for primer: <br/> </p>
| |
| - | <br/>
| |
| - | [[File:Freiburg10 SalI.jpg|800px]]
| |
| - | <br/>
| |
| - | <br/>
| |
| - |
| |
| - | P58 can be used for further RepCap-experiments.
| |
| - |
| |
| - | ===83. Labortag 08.08.2010===
| |
| - |
| |
| - | HT 1080 cells were split and seeded: 3x10^6 cells per into each well of a 6-well plate.
| |
| - |
| |
| - | A431 cells were washed and received new medium.
| |
| - |
| |
| - | There were no clones on the agar plates so they were put back into the 37°C room. Maybe there will be some clones tomorrow.
| |
| - |
| |
| - | ===84. Labortag 09.08.2010===
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Midi-Prep Inoculation</b></p>====
| |
| - | *pAAV_RC
| |
| - | *pAAV_RC_1.2 SDM SalI (P158)
| |
| - | *pAAV_RC_mGMK_TK30 clone 1 (P81)
| |
| - | *pAAV_RC_mGMK_TK30 clone 2 (P82)
| |
| - |
| |
| - | Investigators: Chris W., Patrick
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Continuation: Cloning Rep40 & Rep52 into pSB1C3</b></p>====
| |
| - | <b>Investigator: Patrick </b> <br />
| |
| - | Two Rep52+DMSO clones grew. They were picked and tomorrow there will be a midi prep and a test digestion.
| |
| - | Simultaneously a new approach for cloning Rep40 and Rep52 into pSB1C3 was performed. (see next header)
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Repetition: BioBrick production of Rep40 & Rep52</b></p>====
| |
| - |
| |
| - | '''Investigator: Anna
| |
| - |
| |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': Another approach was done because there were no clones for Rep 40 and only two for Rep 52 (Cloning see 06.08.2010). PCR was performed again with DMSO. Samples from gel extraction are stored in 4°C freezer. </p> <br>
| |
| | <br> | | <br> |
| - | '''PCR
| + | 1% Agarose gel |
| - | | + | |
| - | {| border="1"
| + | |
| - | | '''Ingredients''' || align="right" |'''Rep 40''' ||align="right"|'''Rep52'''
| + | |
| - | |-
| + | |
| - | | 5X Phusion HF buffer || align="right" |10 µl||align="right"|10 µl
| + | |
| - | |-
| + | |
| - | | 10 mM dNTP mix|| align="right" |1µl ||align="right"|1 µl
| + | |
| - | |-
| + | |
| - | | forward primer: O094 || align="right" |2,5µl||align="right"|2,5 µl
| + | |
| - | |-
| + | |
| - | | reverse primer: O0096/ O097 || align="right" |2,5 µl||align="right"|2,5 µl
| + | |
| - | |-
| + | |
| - | | DNA Template (1ng)|| align="right" |1 µl||align="right"| 1 µl
| + | |
| - | |-
| + | |
| - | | DMSO || align="right" |1 µl||align="right"|1 µl
| + | |
| - | |-
| + | |
| - | | Phusion Polymerase|| align="right" |0,5 µl||align="right"| 0,5 µl
| + | |
| - | |-
| + | |
| - | |H<sub>2</sub>O|| align="right" |31,5 µl||align="right"|31,5 µl
| + | |
| - | |-
| + | |
| - | |Total volume|| align="right" |50 µl||align="right"| 50 µl
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| - | | + | <li>1% agarose gel was repared, gel ran for 45 minutes(119 V) |
| - | '''PCR programm
| + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | |'''PCR Program'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time''' || align="right" |'''PCR products
| + | |
| - | |-
| + | |
| - | |1|| align="right" |98 || align="right" |1min|| align="right" |
| + | |
| - | |-
| + | |
| - | |2|| align="right" |98 || align="right" |15s|| align="right" |
| + | |
| - | |-
| + | |
| - | |8x|| align="right" |*** || align="right" |15s|| align="right" |63°C
| + | |
| - | |-
| + | |
| - | |3 || align="right" |72|| align="right" |***|| align="right" |15s
| + | |
| - | |-
| + | |
| - | |4 || align="right" |98|| align="right" | 15s|| align="right" |
| + | |
| - | |-
| + | |
| - | |19x|| align="right" |***|| align="right" |15s|| align="right" |68°C
| + | |
| - | |-
| + | |
| - | |5|| align="right" |72|| align="right" |***|| align="right" |15s
| + | |
| - | |-
| + | |
| - | |6x|| align="right" |72|| align="right" |5min|| align="right" |
| + | |
| - | |-
| + | |
| - | |Hold|| align="right" |4|| align="right" | || align="right" |
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| | | | |
| - | '''Agarose gel
| + | <font color=#FF00FF>Results: unexpected sizes of fragments (see protocol), try again next day with an ethidium bromid gel</font> |
| - | | + | |
| - | 0.5 g Agarose, 50 mL TAE (1 %),3 µL GELRED, at 120 Volt, running time: 45 minutes
| + | |
| - | <br /> | + | |
| - | <br />
| + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample / µl]
| + | |
| - | !Loading dye (6x) / µl
| + | |
| - | !Expected size
| + | |
| - | | + | |
| - | |--
| + | |
| - | |p22 (Rep40)
| + | |
| - | |44 µl
| + | |
| - | |7 µl
| + | |
| - | |940 bp
| + | |
| - | |--
| + | |
| - | |p23 (Rep52)
| + | |
| - | |44 µl
| + | |
| - | |7 µl
| + | |
| - | |~1200 bp
| + | |
| - | |--
| + | |
| - | |}
| + | |
| - | {| align=right
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | *Marker: GeneRuler ladder mix
| + | |
| - | {| border="1"
| + | |
| - | |
| + | |
| - | !Marker /µL
| + | |
| - | !Sample Rep40 /µl
| + | |
| - | !Sample Rep52 /µl
| + | |
| - | |-
| + | |
| - | !Lane
| + | |
| - | |7
| + | |
| - | |51
| + | |
| - | |51
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | | + | |
| - | [[File:Freiburg10 Rep40 Rep52.png|800px]]<br />
| + | |
| - | | + | |
| - | '''Gel extraction<br/>
| + | |
| - | PCR products were eluted with 20 µl BE puffer following the standard protocol.<br/>
| + | |
| - | c(Rep40)= 28,03 ng/µl<br/>
| + | |
| - | c(Rep52)= 121,16 ng/µl<br/>
| + | |
| - | | + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': Digestion was done with the wrong enzymes (EcoRI HF and SpeI), it will be repeated on 11.08. with XbaI and SpeI</p> <br>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Sequence analysis of pSB1C3_mGMK</b></p>==== | + | |
| - | Investigator: <b>Bea </B>
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: mGMK was cloned into the pSB1C3 backbone in order to send it to the parts registry!. The sequence analysis revealed that the mGMK was <b>successfully </b> cloned into pSB1C3 plasmid. This polasmid is in the RFC25 standard.</p>
| + | |
| - | <ul>
| + | |
| - | <b>Results sequencing: Sequence read ok! </b>
| + | |
| - | <br />
| + | |
| - | Primer used: VR-2
| + | |
| - | <br />
| + | |
| - | Comment: A mutation has been found in the pSB1C3 backone which was found generally in the backbone which we received from the iGEM HQ.
| + | |
| - | <br />
| + | |
| - | </ul>
| + | |
| - | [[File:Freiburg10 Sequence analysis of pSB1C3 mGMK 09 08 2010.jpg|1000px]]
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep of pSB1C3_hTERT, pSB1C3_beta-globin_mVenus, pSB1C3_pTAV2(P5), pSB1C3_pAAV_RC(P5TATAles)</b></p>====
| + | |
| - | <b>Investigator: Jessica</b><br>
| + | |
| - | | + | |
| - | '''Bold text'''
| + | |
| - | Mini-Prep following the standart Protokoll
| + | |
| - | <br />
| + | |
| - | <li>P177 = pSB1C3_hTERT clone1 = 163,6 ng/µl
| + | |
| - | <li>P178 = pSB1C3_hTERT clone2 = 312,0 ng/µl
| + | |
| - | <li>P179 = pSB1C3_betaglobin_mVenus clone1 = 237,2 ng/µl
| + | |
| - | <li>P180 = pSB1C3_betaglobin_mVenus clone2 = 228,2 ng/µl
| + | |
| - | <li>P181 = pSB1C3_pTAV2(P5) clone1 = 108,8ng/µl
| + | |
| - | <li>P181 = pSB1C3_pTAV2(P5) clone2 = 140,9 ng/µl
| + | |
| - | <li>P181 = pSB1C3_pAAV_RC(P5TATAless) clone1 = 165,0 ng/µl
| + | |
| - | <li>P181 = pSB1C3_pAAV_RC(P5TATAless) clone2 = 176,6 ng/µl
| + | |
| - | <li>P181 = pSB1C3_pAAV_RC(P5TATAless) clone3 = 176,9 ng/µl
| + | |
| - | <br />
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <b>Test digestion:</b>
| |
| - | <ul>
| |
| - | <li>Perfomed with 10 µL of total volume with all 9 clones</li>
| |
| - | <table border=1 cellpadding=0 cellspacing=0 width=621 style='border-collapse:
| |
| - | collapse;table-layout:fixed;width:466pt'>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt'>
| |
| - | <td height=29 class=xl6724191 width=143 style='height:21.75pt;width:107pt'> </td>
| |
| - | <td class=xl6824191 width=131 style='border-left:none;width:98pt'>Mastermix/µL</td>
| |
| - | <td class=xl6824191 width=87 style='border-left:none;width:65pt'>P176/µL</td>
| |
| - | <td class=xl6824191 width=84 style='border-left:none;width:63pt'>P177/µL</td>
| |
| - | <td class=xl6824191 width=90 style='border-left:none;width:68pt'>P178/µL</td>
| |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P179/µL</td>
| |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P180/µL</td>
| |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P181/µL</td>
| |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P182/µL</td>
| |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P183/µL</td>
| |
| - | <td class=xl6824191 width=86 style='border-left:none;width:65pt'>P184/µL</td>
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>DNA</span></td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>-</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,3</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,3</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,0</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,4</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>6,4</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>5,0</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,2</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,0</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,0</td>
| |
| - |
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| |
| - |
| |
| - | 1'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>BSA (10x)</span></td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | <td rowspan=4 class=xl6524191 style='border-top:none'>3</td>
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| |
| - |
| |
| - | 2'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>Buffer
| |
| - | 4<span style='mso-spacerun:yes'> </span>(10x)</span></td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| |
| - |
| |
| - | 3'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>Enzyme
| |
| - | XbaI<span style='mso-spacerun:yes'> </span></span></td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>5</td>
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| |
| - |
| |
| - | 4'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>Enzyme
| |
| - | AgeI</span></td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>5</td>
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt;mso-yfti-irow:
| |
| - |
| |
| - | 5;mso-yfti-lastrow:yes'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'><span lang=EN-US style='mso-ansi-language:EN-US'>H2O</span></td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>-</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,7</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,7</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,0</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>4,6</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>0,6</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,0</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>2,8</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,0</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>3,0</td>
| |
| - | </tr>
| |
| - | <tr height=29 style='mso-height-source:userset;height:21.75pt'>
| |
| - | <td height=29 class=xl6624191 width=143 style='height:21.75pt;border-top:
| |
| - |
| |
| - | none;width:107pt'>Total volume</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>30</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | <td class=xl6524191 style='border-top:none;border-left:none'>10</td>
| |
| - | </tr>
| |
| - | <tr height=0 style='display:none'>
| |
| - | <td width=143 style='width:107pt'></td>
| |
| - | <td width=131 style='width:98pt'></td>
| |
| - | <td width=87 style='width:65pt'></td>
| |
| - | <td width=84 style='width:63pt'></td>
| |
| - | <td width=90 style='width:68pt'></td>
| |
| - | <td width=86 style='width:65pt'></td>
| |
| - | </tr>
| |
| - | </table>
| |
| - | <br />
| |
| - | </ul>
| |
| - | <ul>
| |
| - | <li>All 9 samples were loaded on a 1% agarose gel</li>
| |
| - | <li>P176 = pSB1C3_hTERT clone1 </li>
| |
| - | <li>P177 = pSB1C3_hTERT clone2 </li>
| |
| - | <li>P178 = pSB1C3_betaglobin clone1</li>
| |
| - | <li>P179 = pSB1C3_betaglobin clone2</li>
| |
| - | <li>P180 = pSB1C3_pTAV2(P5) clone1</li>
| |
| - | <li>P181 = pSB1C3_pTAV2(P5) clone2</li>
| |
| - | <li>P182 = pSB1C3_pAAV_RC(P5TATAless) clone1</li>
| |
| - | <li>P183 = pSB1C3_pAAV_RC(P5TATAless) clone2</li>
| |
| - | <li>P184 = pSB1C3_pAAV_RC(P5TATAless) clone3</li>
| |
| - | </ul>
| |
| - | <br />
| |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| |
| - | !Sample
| |
| - | !Sample/µl]
| |
| - | !Loading dye (6x)/µl
| |
| - | !Expected size 1 (Geneious)
| |
| - | |--
| |
| - | |P176
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |474 bp
| |
| - |
| |
| - | |--
| |
| - | |P177
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |474 bp
| |
| - |
| |
| - | |--
| |
| - | |P178
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |509 bp
| |
| - | |--
| |
| - | |P179
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |509 bp
| |
| - | |--
| |
| - | |P1780
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |164 bp
| |
| - | |--
| |
| - | |P181
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |164 bp
| |
| - | |--
| |
| - | |P182
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |164 bp
| |
| - | |--
| |
| - | |P183
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |164 bp
| |
| - | |--
| |
| - | |P184
| |
| - | |10 µl
| |
| - | |1,6 µl
| |
| - | |164 bp
| |
| - |
| |
| - | |--
| |
| - |
| |
| - | |}
| |
| - | {| align=right
| |
| - | |}
| |
| - | [[File:9 Proben tert, beta, p5.jpg|400px]]
| |
| - | <br />
| |
| - |
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#66bbff;"><b>Comments:</b> hTERT and betaglobin_mVenus is inserted in pSB1C3, pTAV(P5) and pAAV_RC (P5TATAless) will be sequenced</p><br>
| |
| - | Primer: Reverse Primer VR2
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Cell culture</b></p>====
| |
| - | Investigators: Adrian, Kerstin, Patrick
| |
| - | <br />
| |
| - | =====Transfection on HEK293=====
| |
| - | <br />
| |
| - | The motivation: first we want to create new stock of AAV without GOI, the next viral stock is with correct TK/GMK, the last stocks are with different YFP-amounts to check if the amount of GOI is a critical step for transduction effency (transgene expression).
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | Plate || align="right" |pAAV_RC/µg|| align="right" |pHelper/µg|| align="right" |GOI/µg
| |
| - | |-
| |
| - | | 1|| align="right" |3,3 || align="right" | 3,3|| align="right" | no GOI
| |
| - | |-
| |
| - | | 2|| align="right" |6,6 || align="right" | 3,3|| align="right" | no GOI
| |
| - | |-
| |
| - | | 3|| align="right" |3,3 || align="right" | 3,3|| align="right" | 3,3 TK_GMK clone1
| |
| - | |-
| |
| - | | 4|| align="right" |3,3 || align="right" | 3,3|| align="right" | 3,3 TK_GMK clone1
| |
| - | |-
| |
| - | | 5|| align="right" |3,3 || align="right" | 3,3|| align="right" | 3,3 TK_GMK clone2
| |
| - | |-
| |
| - | | 6|| align="right" |3,3 || align="right" | 3,3|| align="right" | 3,3 YFP
| |
| - | |-
| |
| - | | 7|| align="right" |3,3 || align="right" | 3,3|| align="right" | 10 YFP
| |
| - | |-
| |
| - | | 8|| align="right" |3,3 || align="right" | 3,3|| align="right" | 20 YFP
| |
| - | |-
| |
| - | | 9|| align="right" |3,3 || align="right" | 3,3|| align="right" | 40 YFP
| |
| - | |-
| |
| - | | 10|| align="right" |3,3 || align="right" | 3,3|| align="right" | 60 YFP
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | =====Transduction of HT1080=====
| |
| - | <br />Motivation
| |
| - | The Motivation: checking survivalrate of Cells, with either no Virus, virus without GOI and virus with YFP (10µg stock)<br />
| |
| - | The Plan: <br />
| |
| - | Plate I
| |
| - | {| border="1"
| |
| - | | control, no cells || align="right" |no GOI 150µl|| align="right" |10µg 500µl|
| |
| - | |-
| |
| - | | control, no virus|| align="right" |no GOI 300µl|| align="right" | 10µg 750µl|
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | Plate II
| |
| - | {| border="1"
| |
| - | | control, no cells || align="right" |no GOI 150µl|| align="right" |10µg 500µl|
| |
| - | |-
| |
| - | | control, no virus|| align="right" |no GOI 300µl|| align="right" | 10µg 750µl|
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | Plate III
| |
| - | {| border="1"
| |
| - | | control, no cells || align="right" |no GOI 150µl|| align="right" |10µg 500µl|
| |
| - | |-
| |
| - | | control, no virus|| align="right" |no virus|| align="right" | 10µg 750µl|
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | =====Passaging and seeding of A431=====
| |
| - | one 6er dish was prepared for making pictures and one T75 Flask.
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>BioBrick Assembly of pSB1C3_leftITR_CMV and pSB1C3_hGH_rightITR</b></p>====
| |
| - | Investigator: <b>Bea, Achim, Jo</B>
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: In order to proceed with the BioBrick assembly the pasmids pSB1C3_left ITR and pSB1C3_CMV were used and pSB1C3_rigthITR and pSB1C3_hGH were used in order to obatin plasmids with both sequences.</p>
| |
| - |
| |
| - | <gallery widths=400px heights=400px perrow=2 caption="BioBrick assembly of pSB1C3_hGH_rightITR and pSB1C3_leftITR_CMV">
| |
| - | Image:Freiburg10 BioBrick assembbly of pSB1C3 leftITR CMV 09 08 2010.JPG|Cloning strategy of pSB1C3_leftITR CMV
| |
| - | Image:Freiburg10 BioBrick assembbly of pSB1C3 hGH rightITR 09 08 2010.jpg|pSB1C3_hGH_rightITR
| |
| - | </gallery>
| |
| - |
| |
| - | <br />
| |
| - | <br />
| |
| - | <b>Digestion:</b> <br />
| |
| - | {| border="1"
| |
| - | | '''components''' || align="right" |'''pSB1C3_left ITR''' || align="right" |'''pSB1C3_CMV''' || align="right" |'''pSB1C3_right ITR''' || align="right" |''' pSB1C3_hGH'''
| |
| - | |-
| |
| - | | DNA || align="right" |13,4 || align="right" |9,5 || align="right" |13,6 || align="right" |6,6
| |
| - | |-
| |
| - | | BSA (10x) || align="right" |2 || align="right" | 2 || align="right" |2 || align="right" | 2
| |
| - | |-
| |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2 || align="right" |2 || align="right" | 2
| |
| - | |-
| |
| - | |Enzyme1 || align="right" |1 (SpeI) || align="right" |1 (Xba1)|| align="right" |1 (EcoRI) || align="right" |1 (EcoRI)
| |
| - | |-
| |
| - | |Enzyme2 || align="right" |1 (PstI)|| align="right" |1 (PstI) || align="right" |1 (XbaI)|| align="right" |1 (SpeI)
| |
| - | |-
| |
| - | |H2O|| align="right" |0,6 || align="right" |4,5 || align="right" |0,4 || align="right" |7,4
| |
| - | |-
| |
| - | |'''Total volume'''|| align="right" | 20|| align="right" |20 || align="right" | 20|| align="right" |20
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | Preparation of gel:<br />
| |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 115 Volt, running time: 45 minutes
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - | [[Image: Freiburg10 9 8 2010 Digestion of psB1C3 rITR psB1C3 lITR psB1C3 CMV psB1C3 hGH125.jpg|200px]]
| |
| - | <p style="font-size:13px; color:#ff00ff;"><b>Oh, nice picture.. I can recognize many details :)*g* (Hanna) </b> </p>
| |
| - |
| |
| - | Expected sizes of constructs:
| |
| - | *pSB1C3_CMV: 650 bp
| |
| - | *pSB1C3_lITR: 2200 bp
| |
| - | *pSB1C3_hGH: 500 bp
| |
| - | *pSB1C3_rITR: 2200 bp
| |
| - |
| |
| - | The correspnding bands were cut out and Gel-Extraction was performed according to protocol.
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | concentrations measured via NanoDrop:
| |
| - | *pSB1C3_CMV: 13,51 ng/µl
| |
| - | *pSB1C3_lITR: 18,49 ng/µl
| |
| - | *pSB1C3_hGH: 7,22 ng/µl
| |
| - | *pSB1C3_rITR: 20,24 ng/µl
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | <b>Ligation:</b> <br />
| |
| - | For ligation, T4 ligase was used.
| |
| - | <br />
| |
| - | * 1µl T4-Ligase buffer (10x)
| |
| - | * 8µl (Vector + Insert) mix
| |
| - | * 1µl T4-Ligase
| |
| - | Ligation was carried out as following:
| |
| - | * Ligation 1:
| |
| - | **pSB1C3_CMV: 4,4 ng/µl
| |
| - | **pSB1C3_lITR: 3,6 ng/µl
| |
| - | *Ligation 2:
| |
| - | **pSB1C3_hGH: 5,25 µl
| |
| - | **pSB1C3_rITR: 2,75 µl
| |
| - |
| |
| - |
| |
| - | <br /> Incubation for 40 minutes.
| |
| - |
| |
| - | <br />
| |
| - | <b>Transformation:</b><br />
| |
| - | Transformation carried out according to standard protocol (BL21). Cells were plated on an agar plate with chloramphenicol.
| |
| - | <br />
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Repetition: Cloning of SDM SspI and SDM PvuII</b></p>====
| |
| - | Investigator: <b>Stefan</B><br />
| |
| - | <p style="color:red;">Comments: Last week's cloning of SDM_SspI and SDM_PvuII didn't work out, so this is the repetition. In this approach the same restriction enzymes and amounts of DNA were used.</p> <br />
| |
| - | <br />
| |
| - | *Vector: name: pSB1C3_SDM_SspI <b>P125</b>
| |
| - | *Insert: name: pSB1C3_SDM_PvuII <b>P129</b>
| |
| - |
| |
| - | *buffer used: 4
| |
| - | *Restriction-enzymes used:
| |
| - | **BpmI (no. Lab: 144)
| |
| - | **ClaI (no.Lab: 152)
| |
| - | *DNA concentration (P125): 321,9 ng/µl
| |
| - | *DNA concentration (P129): 308,4 ng/µl
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | <b>Digestion:</b> <br />
| |
| - | {| border="1"
| |
| - | | '''components''' || align="right" |'''volume of pSB1C3_SDM_SspI /µl''' || align="right" |'''volume of pSB1C3_SDm_PvuII /µl'''
| |
| - | |-
| |
| - | | DNA || align="right" |4,66 || align="right" |4,87
| |
| - | |-
| |
| - | | BSA (10x) || align="right" |2 || align="right" | 2
| |
| - | |-
| |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2
| |
| - | |-
| |
| - | |BpmI (no.Lab:144)|| align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | |ClaI (no.Lab:152)|| align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | |H2O|| align="right" |9,34 || align="right" |9,13
| |
| - | |-
| |
| - | |'''Total volume'''|| align="right" | 20|| align="right" |20
| |
| - | |}
| |
| - | <br />
| |
| - | <b>Gel extraction:</b> <br />
| |
| - | Preparation of gel:<br />
| |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 115 Volt, running time: 45 minutes
| |
| - | <br />
| |
| - |
| |
| - | Expected sizes of constructs:
| |
| - | *pSB1C3_SDM_SspI: 1819 bp
| |
| - | *pSB1C3_SDM_PvuII: 981 bp
| |
| - |
| |
| - | The correspnding bands were cut out and Gel-Extraction was performed according to protocol.
| |
| - | <br />
| |
| - |
| |
| - | [[File:Freiburg10 pSB1C3 SDM SspI and PvuII 090810.png|500px|left|thumb|]]<br />
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | concentrations measured via NanoDrop:
| |
| - | * Sspl: 19,85 ng/µl
| |
| - | * PvuII: 10,57 ng/µl
| |
| - | <br />
| |
| - |
| |
| - | <b>Ligation:</b> <br />
| |
| - | For ligation, T4 ligase was used.
| |
| - | <br />
| |
| - | * 1µl T4-Ligase buffer (10x)
| |
| - | * 8µl (Vector + Insert) mix
| |
| - | * 1µl T4-Ligase
| |
| - | The Ligation was performed as following:
| |
| - | * SDM_SspI Volume: 4,03 µl
| |
| - | * SDM_PvuII Volume: 3,97 µl
| |
| - | <br />
| |
| - | * 1µl T4-Ligase buffer (10x)
| |
| - | * 8µl (Vector + Insert) mix
| |
| - | * 1µl T4-Ligase
| |
| - | <br /> Incubating for 45 minutes.
| |
| - |
| |
| - | <br />
| |
| - | <b>Transformation:</b><br />
| |
| - | Transformation performed according to the standard protocol (BL21). The cells were plated on a agar plate with chloramphenicol.
| |
| - | <br />
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#ff00ff;"><b>Design and ordering of primer for P40 Promoter BioBrick production</b></p>====
| |
| - | Investigator: <b>Hanna</B><br />
| |
| - | <br/>
| |
| - | In order to generate a P40 promoter BioBrick = "Cap-Promoter", oligos were designed and ordered at Sigma Aldrich:<br/>
| |
| - | [[File:Freiburg10 P40.jpg|450px]]
| |
| - | <br/>
| |
| - |
| |
| - | === 85. labday 10.08.2010===
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Retrafo with RepCap gene (Mr.Gene)</b></p>====
| |
| - | <b>Investigator: Bea</b><br>
| |
| - | <p style="font-size:13px; color:#66bbff;"><b>Comments</b>: We received the rep_cap insert ordered from Mr. Gene today. In order to used and clone the insert into the desired sequences a retrafo has to be performed. </p>
| |
| - | <b> Re-transformation: </b>
| |
| - | <ul>
| |
| - | <li>Construct used: pMA_REP_CAP</li>
| |
| - | <li> We reveived 5 µg (lyophilzied). These were resuspended in 100 µL H<sub>2</sub>O and vortexted.</li>
| |
| - | <li>c = 50 ng/µL</li>
| |
| - | <li>c = Plasmid need to be diluted in order to obatain the final 500pg</li>
| |
| - | <li>Dilution: 1:100</li>
| |
| - | <li>100 µL aliquot of <b>XL1-B</b> were thawed on ice. The proper amount of 1µL was added to the cells.</li>
| |
| - | <li>Transformed cells were plated on LB plates containing ampicillin</li>
| |
| - | </ul>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Continuation: Cloning Rep40 & Rep52 into pSB1C3</b></p>====
| |
| - | A midi prep of the two Rep52+DMSO clones was performed yielding concentrations of 261,35 ng/µl (clone 1) and 240,47 ng/µl (clone 2). Unfortunately we noticed today that we should not use EcoRI to clone Rep52 into pSB1C3 because EcoRI cuts two times within the sequence of Rep52.
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Midi-Prep of pAAV_RC, pAAV_RC_1.2 SDM SalI (P158), pAAV_RFC25_mGMK_TK30 clone 1 (P81) and 2 (P82)</b></p>====
| |
| - | Investigators: Patrick, Chris <br>
| |
| - |
| |
| - | The Midi-Preps were performed according to the standard protocol yielding the following concentrations:
| |
| - |
| |
| - | {| border="1"
| |
| - | | pAAV_RC || align="right" |P81a|| align="right" |P81b|| align="right" |P82a|| align="right" |P82b|| align="right" |P158a|| align="right" |P158b
| |
| - | |-
| |
| - | | 1348,49 ng/µl|| align="right" |1783,86 ng/µl|| align="right" | 2096,25 ng/µl|| align="right" |1915 ng/µl|| align="right" |1469,35 ng/µl|| align="right" |1834,25 ng/µl|| align="right" |1771,27 ng/µl
| |
| - | |-
| |
| - | |}
| |
| | <br> | | <br> |
| - | The annotations a and b were added because two midi-preps of P81, P82 and P158 were carried out.
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Production of chemical competent E.coli</b></p>==== |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Results of sequencing pSB1C3_pTAV2(P5) and Psb1C3_pAAV_RC(P5TATAless)</b></p>====
| + | <p><b>Investigators: Patrick and Jessica</b></p> |
| - | <b>Investigator: Jessica</b><br>
| + | |
| - | | + | |
| - | [[image:mistake.png|thumb|right]]
| + | |
| - | @Jessi: Which clones are ok, which not?? text does not correspond to pictures! (Bea)
| + | |
| - | <br />
| + | |
| - | [[File:Freiburg10_Sequence_alignment_P5 mit TATA clone1neu.jpg|500px|left|thumb|]]
| + | |
| | <br> | | <br> |
| | + | competent E.coli XL-1-blue and competent E.coli BL21 produced according to the standard-protocoll [[Media:production of competent E.coli.pdf]]<br> |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | sequencing of '''P180''' was successful, P5 with TATAbox is in pSB1C3
| |
| - | [[File:Freiburg10_Sequence_alignment_P5TATAless clone2neu.jpg|500px|left|thumb|]]
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | sequencing of '''P183''' was successful but there is a G missing --> plamid is thrown away
| |
| - | [[File:Freiburg10_Sequence_alignment_P5TATAless clone3.jpg|500px|left|thumb|]]
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | sequencing of '''P184''' was successful, P5 is in without TATAbox. this plasmid can be used. <br>
| |
| - | <br>
| |
| - | '''P181''' and '''P180''' are thrown away because of the result of the test digestion.
| |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Repetition: BioBrick production of Rep40 and Rep 52</b></p>====
| |
| - | Investigator: <b>Anna</B><br />
| |
| | | | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': PCR was repeated once again (that was bad!), because digestion was done with the wrong enzymes (EcoRI HF and SpeI). There are some differences in the PCR programm in comparison to the last (see 09.10.)</p> <br> | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Design of MCS-Oligos</b></p>==== |
| | + | <p><b>Investigators: Bea, Adrian, Hanna, Sven</b></p> |
| | <br> | | <br> |
| - | '''PCR
| + | In order to replace the multiple cloning site of the pAAV_MCS vector from Stratagene by RFC25, two oligos were designed. After their hybridization the overhangs correspond to ClaI- and BglII restriction sites. A digestion with BglII and ClaI will be performed with pAAV-MCS and then will be ligated with the designed oligos.<br> |
| | + | The oligos (see link) were ordered at Sigma-Aldrich. Estimated shipment: 31.05.2010 .<br> |
| | | | |
| - | {| border="1"
| |
| - | | '''Ingredients''' || align="right" |'''Rep 40''' ||align="right"|'''Rep52'''
| |
| - | |-
| |
| - | | 5X Phusion HF buffer || align="right" |10 µl||align="right"|10 µl
| |
| - | |-
| |
| - | | 10 mM dNTP mix|| align="right" |1µl ||align="right"|1 µl
| |
| - | |-
| |
| - | | forward primer: O094 || align="right" |2,5µl||align="right"|2,5 µl
| |
| - | |-
| |
| - | | reverse primer: O0096/ O097 || align="right" |2,5 µl||align="right"|2,5 µl
| |
| - | |-
| |
| - | | DNA Template 1:100 (1ng)|| align="right" |1 µl||align="right"| 1 µl
| |
| - | |-
| |
| - | | DMSO || align="right" |1 µl||align="right"|1 µl
| |
| - | |-
| |
| - | | Phusion Polymerase|| align="right" |0,5 µl||align="right"| 0,5 µl
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| align="right" |31,5 µl||align="right"|31,5 µl
| |
| - | |-
| |
| - | |Total volume|| align="right" |50 µl||align="right"| 50 µl
| |
| - | |}
| |
| - | <br>
| |
| | | | |
| - | '''PCR programm
| + | [[File:Freiburg10 Oligos MCS RFC25 for pAAV.pdf]] |
| | | | |
| - | {| border="1"
| + | ===15. Labortag 26.05.2010=== |
| - | |'''PCR Program'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time''' || align="right" |'''PCR products
| + | |
| - | |-
| + | |
| - | |1|| align="right" |98 || align="right" |1min|| align="right" |
| + | |
| - | |-
| + | |
| - | |2|| align="right" |98 || align="right" |15s|| align="right" |
| + | |
| - | |-
| + | |
| - | |8x|| align="right" |*** || align="right" |15s|| align="right" |'''62,5°C
| + | |
| - | |-
| + | |
| - | |3 || align="right" |72|| align="right" |***|| align="right" |'''25s
| + | |
| - | |-
| + | |
| - | |4 || align="right" |98|| align="right" | 15s|| align="right" |
| + | |
| - | |-
| + | |
| - | |19x|| align="right" |***|| align="right" |15s|| align="right" |'''67°C
| + | |
| - | |-
| + | |
| - | |5|| align="right" |72|| align="right" |***|| align="right" |'''25s
| + | |
| - | |-
| + | |
| - | |6x|| align="right" |72|| align="right" |5min|| align="right" |
| + | |
| - | |-
| + | |
| - | |Hold|| align="right" |4|| align="right" | || align="right" |
| + | |
| - | |}
| + | |
| - | <br>
| + | |
| | | | |
| - | '''Agarose gel
| + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Oligos (PstI + MCS)</b></p>==== |
| | | | |
| - | 0.5 g Agarose, 50 mL TAE (1 %),3 µL GELRED, at 120 Volt, running time: 45 minutes
| + | <p><b>Investigators: Bea, Hanna, Jessica</b></p> |
| - | <br /> | + | |
| - | <br /> | + | |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| + | |
| - | !Sample
| + | |
| - | !Sample / µl]
| + | |
| - | !Loading dye (6x) / µl
| + | |
| - | !Expected size
| + | |
| | | | |
| - | |--
| |
| - | |p22 (Rep40)
| |
| - | |50 µl
| |
| - | |8 µl
| |
| - | |940 bp
| |
| - | |--
| |
| - | |p23 (Rep52)
| |
| - | |50 µl
| |
| - | |8 µl
| |
| - | |~1200 bp
| |
| - | |--
| |
| - | |}
| |
| - | {| align=right
| |
| - | |}
| |
| - | <br />
| |
| - | *Marker: GeneRuler ladder mix
| |
| - | {| border="1"
| |
| - | |
| |
| - | !Marker /µL
| |
| - | !Sample Rep40 /µl
| |
| - | !Sample Rep52 /µl
| |
| - | |-
| |
| - | !Lane
| |
| - | |7
| |
| - | |58
| |
| - | |58
| |
| - | |-
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | [[File:Freiburg10_Rep40_52.jpg|500px|left|thumb|]]
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - |
| |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': Digestion of the samples will be done tomorrow with XbaI and SpeI.</p> <br>
| |
| - | <br />
| |
| - |
| |
| - | '''Gelextraction
| |
| - |
| |
| - | Elution was done with 20 µl Pe buffer following the standard protocol.<br />
| |
| - |
| |
| - | c(Rep40)= 23,1 ng/µl<br />
| |
| - | c(Rep52)= 78,0 ng/µl
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Preparation of competent XL1blue and testtrafo</b></p>====
| |
| - | <b>Investigator: Jessica</b><br>
| |
| - | preparation of competent E.coli XL1blue was made according to the standardprotocol (91 eppis with '''60µl''') they are in the green boxes in -80°C<br>
| |
| - | testtrafo was made
| |
| - | <ul><li>one plate with 50pg/µl</li>
| |
| - | <li>one plate with 100pg/µl</li>
| |
| - | </ul>
| |
| - | ...trafo is failed because amp was missing on the plates (resistence in pUC). trafo will be repeated on aug 11th by Tobi...
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Repetition: Mini-Prep and test digestion of pSB1C3_SDM_SspI/PvuII</b></p>====
| |
| - | '''Investigator: Adrian, Stefan'''<br>
| |
| - | <br>
| |
| - | <p style="font-size:18px; font-weight: bold; color:#66bbff;"><u>Plasmid Mini-Prep</u></p>
| |
| - | *vector name: pSB1C3_SDM_SspI/PvuII
| |
| - | <br />
| |
| - | <u>Glycerol Stocks</u> <br>
| |
| - | pSB1C3_SDM_SspI/PvuII
| |
| - | Only clone 1 grew, therefore there is just one stock = B159
| |
| - |
| |
| - |
| |
| - |
| |
| - | <p style="font-size:15px; font-weight: bold; color:#66bbff;">Test digestion</p>
| |
| - | *buffer used: 2
| |
| - | *Restriction-enzymes used:
| |
| - | **Enzyme 1 SspI
| |
| - | **Enzyme 2 PvuII
| |
| - | *Plasmid: pSB1C3_SDM_SspI/PvuII:
| |
| - | **Plasmid-Number: P185; DNA concentration: 47,9 ng/µL <br />
| |
| - |
| |
| - | <p style="font-size:13px; color:#ff0000;">comment: DNA was eluted into previously used tube, therefore contamination is possible.</p> <br>
| |
| - |
| |
| - |
| |
| - | <br />
| |
| - |
| |
| - | {| border="1"
| |
| - | | align="left" | '''Components''' ||align="left"| <b>P185</b> Volume/µL ||align="left"| <b>P51.1</b> Volume/µL
| |
| - | |-
| |
| - | | align="left" | DNA ||align="left"| 18,1 ||align="left"| 6,8
| |
| - | |-
| |
| - | | align="left" | BSA (10x) ||align="left"| 2,5 ||align="left"| 2,5
| |
| - | |-
| |
| - | | align="left" | Buffer no. 2 (10x) ||align="left"| 2,5 ||align="left"| 2,5
| |
| - | |-
| |
| - | | align="left" | Enzyme 1 SspI ||align="left"| 0.5 ||align="left"| 0.5
| |
| - | |-
| |
| - | | align="left" | Enzyme 2 PvuII ||align="left"| 0.5 ||align="left"| 0.5
| |
| - | |-
| |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 0,9 ||align="left"| 12,2
| |
| - | |-
| |
| - | | align="left" | '''Total volume''' ||align="left"| <b>10</b> ||align="left"| <b>10</b> |
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | *Incubation: 45 min
| |
| - | <br />
| |
| - | <p style="font-size:15px; font-weight: bold; color:#66bbff;">Agarose-Gel:</p>
| |
| - | <br />
| |
| - | 0.4 g Agarose, 50 mL <b>TAE</b>, 3 µL GELRED, at 115 Volt, running time: 45 minutes
| |
| - | <br />
| |
| - |
| |
| - | {| align=right
| |
| - | |}
| |
| - | <br />
| |
| - | *Marker: GeneRuler ladder mix
| |
| - | {| border="1"
| |
| - | |
| |
| - | !Marker /µL
| |
| - | !Sample P185 /µL
| |
| - | !Sample P51.1 /µL
| |
| - |
| |
| - | |-
| |
| - | !Lane
| |
| - | |7
| |
| - | |17
| |
| - | |17
| |
| - | |-
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | One cut is expected (PvuII in CFP).<br>
| |
| - | [[File:Freiburg10 SspI PvuII test digestion.png|300px]] <br>
| |
| - | <br>
| |
| - | <b>Comment: There are 6 bands now. This test digestion again looks very different from that of P157.2 done by Jessica (lab day 82). Results should be discussed tomorrow.</b><br>
| |
| - | <br>
| |
| - |
| |
| - | <br/>
| |
| - | <br/>
| |
| - |
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Picking clones of pSB1C3_SDM_SspI/PvuII</b></p>====
| |
| - | <p><b>Investigator: Stefan</b></p>
| |
| - | <p style="font-size:13px; color:#66bbff;"><b>Comments:</b> Test digestion didn't work out, three additional clones were picked from the plate we get from yesterday's Trafo to retry tomorrow.</p>
| |
| | <ul> | | <ul> |
| - | <li>Bacterial strain used: BL21</li> | + | <li>Repeat cloning of pAAV-MCS + pCMV_mVenus_YFP --> <b>Goal: pAAV_mVenus_YFP</b><br> |
| - | <li>Inoculating of 10 mL DYT medium containing 10µL chloramphenicol</li>
| + | |
| - | <li> Put in 37°C room on shaker at 20.45hours.</li>
| + | |
| - | </ul><br><br>
| + | |
| - | | + | |
| - | === 86. labday 11.08.2010===
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Biobrick Assembly:Picking Clones, Miniprep, Testdigestion of CMV&lITR/ hGH & rITR</b></p>====
| + | |
| - | Investigator: <b>Chris L., Achim</B>
| + | |
| - | Test Digestion showed that the cloning was succesful.
| + | |
| - | [[Image:Freiburg10_assembly1.jpg]]
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | NanoDrop concentrations:
| + | |
| - | *hGH_rITR1:105,15 ng/µl
| + | |
| - | *hGH_rITR2:96.97 ng/µl
| + | |
| - | *CMV_lITR1:231.61 ng/µl
| + | |
| - | *CMV_lITR2:129.19 ng/µl
| + | |
| - | <br />
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Biobrick Assembly: bSB1C3_lITR_CMV & pSB1C3_betaglobin_mVenus</b></p>====
| + | |
| - | Investigator: <b>Chris L., Achim</B>
| + | |
| - | <br /> | + | |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: In order to proceed with the BioBrick assembly the plasmids pSB1C3_leftITR_CMV was cut with SpeI and PstI, pSB1C3_betaglobin_mVenus was cut with XbaI and PstI. The insert fragment containing betaglobin_mVenus was ligated to the vector fragment containing the leftITR_CMV promotor.</p>
| + | |
| - | | + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <b>Digestion:</b> <br />
| + | |
| - | {| border="1"
| + | |
| - | | '''components''' || align="right" |'''pSB1C3_leftITR_CMV''' || align="right" |'''pSB1C3_betaglobin_mVenus'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |5,2 || align="right" |8,4
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |2 || align="right" | 2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2
| + | |
| - | |-
| + | |
| - | |Enzyme1 || align="right" |1 (SpeI) || align="right" |1 (Xba1)
| + | |
| - | |-
| + | |
| - | |Enzyme2 || align="right" |1 (PstI)|| align="right" |1 (PstI)
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |8,8 || align="right" |5,6
| + | |
| - | |-
| + | |
| - | |'''Total volume'''|| align="right" | 20|| align="right" |20
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | | + | |
| - | Preparation of gel:<br />
| + | |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 115 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | | + | |
| - | Expected sizes of constructs:
| + | |
| - | *pSB1C3_leftITR_CMV: 2800 bp
| + | |
| - | *pSB1C3_betaglobin_mVenus: 1200 bp, 2000 bp
| + | |
| - | | + | |
| - | The corresponding bands were cut out and Gel-Extraction was performed according to protocol.
| + | |
| - | <br />
| + | |
| - | | + | |
| - | concentrations measured via NanoDrop:
| + | |
| - | *pSB1C3_leftITR_CMV: 55,5 ng/µl
| + | |
| - | *pSB1C3_betaglobin_mVenus:: 10,4 ng/µl
| + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Continuation of Cloning of Rep40/52</b></p>====
| + | |
| - | | + | |
| - | '''Investigator: Anna
| + | |
| - | | + | |
| - | | + | |
| - | *Digestion of PCR products and vector:
| + | |
| - | | + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | '''components''' || align="right" |'''PCR product /µl''' || align="right" |'''vector /µl'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |19 || align="right" |10
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |3 || align="right" | 3
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |3 || align="right" |3
| + | |
| - | |-
| + | |
| - | |Enzyme '''XbaI || align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |Enzyme '''SpeI || align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |3 || align="right" |12
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 30|| align="right" |30
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': This time digestion was done with XbaI and SpeI (means it has to be checked if the inserts are cloned into the vector in the right orientation). </p> <br>
| + | |
| - | | + | |
| - | *Purification of Rep40 and Rep52:
| + | |
| - | For the purification 95 µl of buffer PBI was used.
| + | |
| - | | + | |
| - | c(Rep40)= 12,5 ng/µl <br />
| + | |
| - | c(Rep52)= 51,8 ng/µl <br />
| + | |
| - | | + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | *Gelextraction of pSB1C3_RFC25_CFP:
| + | |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 120 Volt, running time:55 <br />
| + | |
| - | 5µl loading dye (6x) for the sample, Marker: GeneRuler ladder mix (Fermentas)
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | | + | |
| - | [[file:Digestion of pSB1C3_2.jpg]]
| + | |
| - | <br />
| + | |
| - | | + | |
| - | | + | |
| - | c(pSB1C3)= 10,59 ng/µl <br />
| + | |
| - | c(pSB1C3_2)= 4,25 ng/µl
| + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | *Ligation of PCR products and vector:
| + | |
| - | | + | |
| - | For the Ligation 1µl T4 buffer (2x) and 1µl T4 ligase were used.
| + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | ''' ''' || align="right" |'''Sample-no.'''|| align="right" |'''vector /µl''' || align="right" |'''insert /µl'''
| + | |
| - | |-
| + | |
| - | | pSB1C3 + Rep40 || align="right" |1 || align="right" |3,65 || align="right" |4,35
| + | |
| - | |-
| + | |
| - | | pSB1C3 + Rep52 || align="right" |2 || align="right" |5,87 || align="right" |2,13
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 8|| align="right" | || align="right" |
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | | + | |
| - | *Transformation:
| + | |
| - | | + | |
| - | The transformation was done following the standard protocol using B21 cells.
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>PCR of bla_14FM and ligation into pSB1C3_SDM_SspI and pSB1C3_SDM_PvuII for virobrick-production T</b></p>====
| + | Cloning did not work (see lab day 25.05.2010) either with NotI or with NotI-HF. Gel did not show any proper band at the expected size. <br> |
| - | <br/> | + | There will be used EtBr instead of gelred in the agarose gel and the incubation time of digestion will be prolonged. Further details are followed. <br> |
| - | Investigator: Anissa,Kerstin<br />
| + | NOTE: There are three restriction sites of NotI in the pCMV-mVenus_YFP. If cloning with NotI, the polyA hGH signal will be deleted. therefore cloning with NotI is '''not''' possible . |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': Because it still is unclear if SDM of pSB1C3_SspI_PvuII has worked, we decided to use the one time mutagenisised vector pSB1C3_SDM_SspI, to clone bla into it and to make the second mutation of PvuII after bla is inserted. . </p> <br>
| + | |
| | <br> | | <br> |
| - | *'''PCR:
| |
| - | (was performed following the standard protocol)
| |
| - | <br>
| |
| - | {| border="1"
| |
| - | | '''Ingredients''' || align="right" |'''v(pTB106_bla14FM) - DMSO / µl''' || align="right" |'''v(pTB106_bla14FM) + DMSO / µl'''
| |
| - | |-
| |
| - | | 5X Phusion HF buffer || align="right" |10 || align="right" |10
| |
| - | |-
| |
| - | | 10 mM dNTP mix|| align="right" |1 || align="right" |1
| |
| - | |-
| |
| - | | forward primer: O116(P extreme f)|| align="right" |2,5 || align="right" |2,5
| |
| - | |-
| |
| - | | reverse primer: O117 (P extreme r) || align="right" |2,5 || align="right" |2,5
| |
| - | |-
| |
| - | | DNA Template 1ng || align="right" |5,3 µl || align="right" |5,3 µl
| |
| - | |-
| |
| - | | DMSO (1%)|| align="right" | 0,5 || align="right" | 0,5
| |
| - | |-
| |
| - | | Phusion Polymerase|| align="right" |0,5 || align="right" |0,5
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| align="right" |28,2 || align="right" |27,7
| |
| - | |-
| |
| - | |Total volume|| align="right" |50 || align="right" |50
| |
| - | |}
| |
| | <br> | | <br> |
| | + | <li>Test digestion of pCMV-mVenusYFP with PstI and MluI <br> |
| | | | |
| - | *''' PCR program:
| + | <b>Digestion</b> |
| - | | + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | |'''PCR Program'''|| align="right" |'''temperature/ °C''' |'''time'''
| + | |
| - | |-
| + | |
| - | |1|| align="right" |98 || align="right" | 1'
| + | |
| - | |-
| + | |
| - | |2|| align="right" |98 || align="right" | 15''
| + | |
| - | |-
| + | |
| - | |8x|| align="right" |61 || align="right" | 25''
| + | |
| - | |-
| + | |
| - | |3 || align="right" |72 || align="right" | 15''
| + | |
| - | |-
| + | |
| - | |4 || align="right" |98 || align="right" | 15''
| + | |
| - | |-
| + | |
| - | |5|| align="right" |72 || align="right" | 15''
| + | |
| - | |-
| + | |
| - | |1x|| align="right" |72 || align="right" | 5'
| + | |
| - | |-
| + | |
| - | |Hold|| align="right" |4
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| - | | + | <ul> |
| - | [[File:Freiburg10 pcr bla14FM.JPG|300px]]
| + | <li>experiment date: 26.05.2010 </li> |
| - | | + | <li>plasmid: pCMV_mVenus_YFP; number: P5; production date: 21.05.2010/ pCMV-MSC; number: P2; production date: </li> |
| - | | + | <li>buffer used: 3/4; Restriction-enzymes used: Enzyme PstI (no. Lab:48) and Enyzme MluI (no. Lab:40) / NotI HF (no. Lab:159) </li> |
| - | | + | <li>DNA concentration plasmid: P5 477,3 ng/µl / P2 550ng/µl </li> |
| - | *'''Digestion PCR product:
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | * DNA-Concentration: 4,2 ng/µl
| + | |
| - | | + | |
| | | | |
| | {| border="1" | | {| border="1" |
| - | |'''components'''|| align="right" |'''volume / µl''' | + | | components || align="right" | V (pCMV_mVenus_YFP)/ µl ||align="right"| V(pCMV_MCS) / µl |
| | |- | | |- |
| - | | DNA || align="right" | 28,5 | + | | DNA || align="right" | 4,2||align="right"|3,6 |
| | |- | | |- |
| - | | BSA 100x || align="right" | 0,4 | + | | BSA (10x) || align="right" |2||align="right"|2 |
| | |- | | |- |
| - | |Buffer: 4 10x || align="right" | 4 | + | | Buffer 3 (10x)|| align="right" |2||align="right"|2 |
| | |- | | |- |
| - | | Enzyme 1 (XbaI) || align="right" | 1 | + | |Enzyme: PstI/NotI HF (no.Lab:48/159)|| align="right" |1||align="right"|2 |
| | |- | | |- |
| - | | Enzyme 2 (PstI) || align="right" | 1 | + | |Enzyme: MluI (no.Lab:40)|| align="right" |1||align="right"|- |
| | |- | | |- |
| - | | H<sub>2</sub>O || align="right" | 5,1 | + | |H2O|| align="right" |9,8||align="right"|10,4 |
| - | |- | + | |
| - | | Total volume || align="right" | 40 | + | |
| | |- | | |- |
| | + | |'''Total volume'''|| align="right" |<b>20</b>||align="right"|<b>20</b> |
| | |} | | |} |
| - |
| |
| - | * ''' Purification of PCR product: (QlAquick Gel Extraction Kit)
| |
| - |
| |
| - |
| |
| - | preparation of purification was made according to the standardprotocol
| |
| - |
| |
| - |
| |
| - |
| |
| - | <p style="font-size:13px; color:#FF0033;">'''''Comments''''': The wrong enzymes were used. Experiment has to be done tomorrow.</p> <br>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Test digestion pSB1C3_CFP_SDM SspI and PvuII </b></p>====
| |
| - | '''Investigator: Stefan'''<br>
| |
| - | *buffer used: 2; Restriction-enzymes used: Enzyme 1: ClaI ; Enzyme 2: BpmI ; Enzyme 3: SspI ; Enzyme 4: PvuII
| |
| - | <ul><li>Plasmids:</li>
| |
| - | <ul><li>pSB1C3_SDM_SspI/PvuII '''P185''' (first tree lanes, cut with different enzymes)</li>
| |
| - | <li>pSB1C3_RFC25_longlinker '''P105'''</li>
| |
| - | <li>pSB1C3_CFP '''P51.1'''</li>
| |
| - | </ul>
| |
| - | </ul>
| |
| - |
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | align="left" | '''Components''' ||align="left"| '''P185/µL''' ||align="left"| '''P185/µL''' ||align="left"| '''P185/µL''' ||align="left"| '''P105/µL''' ||align="left"| '''P51.1/µL'''
| |
| - | |-
| |
| - | | align="left" | DNA ||align="left"| 18||align="left"| 18||align="left"| 18||align="left"| 4,8||align="left"|6,8
| |
| - | |-
| |
| - | | align="left" | BSA (10x) ||align="left"| 2,5||align="left"| 2,5||align="left"| 2,5||align="left"| 2,5||align="left"| 2,5
| |
| - | |-
| |
| - | | align="left" | Buffer 2 (10x) ||align="left"| -||align="left"| -||align="left"| -||align="left"| 2,5||align="left"| 2,5
| |
| - | |-
| |
| - | | align="left" | Buffer 4 (10x) ||align="left"| 2,5||align="left"| 2,5||align="left"| 2,5||align="left"| -||align="left"| -
| |
| - | |-
| |
| - | | align="left" | Enzyme 1 ClaI (152) ||align="left"| 0,5||align="left"| 0,5||align="left"| 0,5||align="left"| -||align="left"| -
| |
| - | |-
| |
| - | | align="left" | Enzyme 2 BpmI (144) ||align="left"| 0,5||align="left"| 0,5||align="left"| 0,5||align="left"| -||align="left"| -
| |
| - | |-
| |
| - | | align="left" | Enzyme 3 SspI (68) ||align="left"| -||align="left"| 0,5||align="left"| -||align="left"| 0,5||align="left"| 0,5
| |
| - | |-
| |
| - | | align="left" | Enzyme 4 PvuII (50) ||align="left"| -||align="left"| -||align="left"| 1||align="left"| 0,5||align="left"| 0,5
| |
| - | |-
| |
| - | | align="left" | H<sub>2</sub>O ||align="left"| 1||align="left"| -||align="left"| 0,5||align="left"| 14,2||align="left"| 12,2
| |
| - | |-
| |
| - | | align="left" | '''Total volume''' ||align="left"| 25||align="left"| 25||align="left"| 25||align="left"| 25||align="left"| 25
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | *Incubation: 75 minutes<br>
| |
| - | '''Agarosegel'''
| |
| - | <br />
| |
| - | 0.5 g Agarose, 50 ml TAE, 3 µL GELRED, at 115 Volt, running time: 60 minutes
| |
| - | <br />
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - | <br />
| |
| - | [[File:Freiburg10 SspI PvuII ClaI BpmI later.png|500px|left|thumb|]]
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| - | <br>
| |
| | <br> | | <br> |
| - | | + | <li> Incubation: 1 h at 37°C |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| | | | |
| | <br> | | <br> |
| - | comments: Test digestion of pSB1C3_SDM_SspI/PvuII with ClaI, BpmI and SspI looks the same as with ClaI and BpmI. Therefore we can assume that there is no additional restriction site within the vector. But still the bands above 3000 bp cannotz be explained. A new test digestion will be performed tomorrow to check the size of the vector.<br />
| + | 1% Agarose gel |
| - | The approach cut with ClaI, BpmI and PvuII looks strange. Propably, PvuII does not work correctly. New PvuII-HF was ordered and delivered so this can be verified tomorrow.<br />
| + | |
| - | pSB1C3_RFC25_longlinker digested with SspI and PvuII seems to be not completely digested This could correspond to non-working PvuII.
| + | |
| - | pSB1C3_CFP looks like expected.
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>pSB1C3_SDM_Ssp1 (P125) sent for sequenzing</b></p>====
| + | |
| - | <br/>
| + | |
| - | Investigator: Stefan<br />
| + | |
| - | | + | |
| - | comment: To verify the correct deletion of the SspI restriction site it was sent for sequenzing.
| + | |
| - | | + | |
| - | Primer used: GATC_std_pTeSp-2
| + | |
| - | | + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Picking clones of pSB1C3_SDM_SspI/PvuII (P185)</b></p>====
| + | |
| - | <br/>
| + | |
| - | Investigator: Adrian<br />
| + | |
| - | | + | |
| - | comment: pSB1C3_SDM_SspI/PvuII (P185) is empty. For plasmid production DYT + Cm was inoculated from the glycerol stock (B159).<br>
| + | |
| - | | + | |
| - | === 87. labday 12.08.2010===
| + | |
| - | ====<p style="font-size:15px; background-color:#FF0033;"><b>XL1blue are ready to use</b></p>====
| + | |
| - | '''Investigator: Jessica'''
| + | |
| - | <br>there are 60µl aliquots to use for one trafo
| + | |
| - | | + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep of pSB1C3_SDM_SspI/PvuII and pMA_RC_insert</b></p>====
| + | |
| - | <br/>
| + | |
| - | Investigator: Stefan<br />
| + | |
| - | | + | |
| - | Gycerol stock was prepared for pMA_RC_insert and labeled B167. No glyerol stock for pSB1C3_SDM_SspI/PvuII because cells from earlier stock werer used for Mini-Prep.
| + | |
| - | | + | |
| - | Miniprep was performed according to protocol.<br />
| + | |
| - | | + | |
| - | Samples were labeled:<br />
| + | |
| - | *pSB1C3_SDM_SspI/PvuII = P185.1
| + | |
| - | *pMA_RC_insert = P190
| + | |
| - | Concentrations:
| + | |
| - | *pSB1C3_SDM_SspI/PvuII (P185.1): 257,2 ng/µl
| + | |
| - | *pMA_RC_insert (P190): 339,5 ng/µl
| + | |
| - | <br />
| + | |
| - | No test digestion was performed.
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Biobrick Assembly: pSB1C3_lITR_CMV & pSB1C3_betaglobin_mVenus</b></p>====
| + | |
| - | Investigator: <b>Chris L., Achim</B>
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: In order to proceed with the BioBrick assembly the plasmids pSB1C3_leftITR_CMV was cut with SpeI and PstI, pSB1C3_betaglobin_mVenus was cut with XbaI and PstI. The insert fragment containing betaglobin_mVenus was ligated to the vector fragment containing the leftITR_CMV promotor.</p>
| + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | <b>Ligation:</b> <br />
| + | |
| - | For ligation, T4 ligase was used.
| + | |
| - | <br />
| + | |
| - | * 1µl T4-Ligase buffer (10x)
| + | |
| - | * 8µl (Vector + Insert) mix
| + | |
| - | * 1µl T4-Ligase
| + | |
| - | Ligation was carried out as following:
| + | |
| - | *pSB1C3_leftITR_CMV: 6,98 µl
| + | |
| - | *pSB1C3_betaglobin_mVenus: 1,02 µl
| + | |
| - | | + | |
| - | <br /> Incubation for 40 minutes.
| + | |
| - | | + | |
| - | *Transformation:
| + | |
| - | | + | |
| - | The transformation was done following the standard protocol using XL1B cells.
| + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Repetition of cloning of bla14FM_viralbrick_MCS into pSB1C3_SDM_SspI</b></p>====
| + | |
| - | <br/>
| + | |
| - | Investigator: Anissa<br />
| + | |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>:Yesterday the pcr-product of bla14FM was cut with the wrong enzymes, so no overhangs could result. Today it will be repeated with the rihgt ones (SpeI and NgomIV) .</p>
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | *'''Digestion of the PCR product bla14FM for 2 h :
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | * DNA-Concentration: 10,2 ng/µl
| + | |
| - | | + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | |'''components'''|| align="right" |'''volume / µl'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" | 18
| + | |
| - | |-
| + | |
| - | | BSA 100x || align="right" | 3
| + | |
| - | |-
| + | |
| - | |Buffer: 4 10x || align="right" | 3
| + | |
| - | |-
| + | |
| - | | Enzyme 1 (NgomIV) || align="right" | 1
| + | |
| - | |-
| + | |
| - | | Enzyme 2 (SpeI) || align="right" | 1
| + | |
| - | |-
| + | |
| - | | H<sub>2</sub>O || align="right" | 4
| + | |
| - | |-
| + | |
| - | | Total volume || align="right" | 30
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | | + | |
| - | * ''' Purification of PCR product: (QlAquick Gel Extraction Kit)
| + | |
| - |
| + | |
| - | | + | |
| - | preparation of purification was made according to the standardprotocol
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | *'''Digestion of vector backbone pSB1C3_SDM_SspI for 2 h:
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | *1 µg vector has been used
| + | |
| - | | + | |
| - | {| border="1"
| + | |
| - | |'''components'''|| align="right" |'''volume / µl'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" | 3,12
| + | |
| - | |-
| + | |
| - | | BSA 100x || align="right" | 1,5
| + | |
| - | |-
| + | |
| - | |Buffer: 4 10x || align="right" | 1,5
| + | |
| - | |-
| + | |
| - | | Enzyme 1 (NgomIV) || align="right" | 1
| + | |
| - | |-
| + | |
| - | | Enzyme 2 (SpeI) || align="right" | 1
| + | |
| - | |-
| + | |
| - | | H<sub>2</sub>O || align="right" | 6,88
| + | |
| - | |-
| + | |
| - | | Total volume || align="right" | 15
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | *the cut vector was loaded on a preperative 1% agarose gel and the gel was running for 45 minutes
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | [[File:Freiburg10 pSB1C3 SDM SspI cut.png|400px]]
| + | |
| - | <br/>
| + | |
| - | *gel was cut and gelextraction was performed according the qiagen standard protocol.
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | *'''Ligation of pSB1C3_SDM_SspI and bla14FM:
| + | |
| - | <br/>
| + | |
| - | {| border="1"
| + | |
| - | |'''components'''|| align="right" |'''volume / µl'''
| + | |
| - | |-
| + | |
| - | |t4 buffer (10X) || align="right" | 1
| + | |
| - | |-
| + | |
| - | | t4 Ligase || align="right" | 1
| + | |
| - | |-
| + | |
| - | |vector DNA || align="right" | 2,72
| + | |
| - | |-
| + | |
| - | | Insert || align="right" | 5,28
| + | |
| - | |-
| + | |
| - | |}
| + | |
| - | <br/>
| + | |
| - | *'''Transformation: pSB1C3_SDM_SspI_bla14FM was transformed into BL21 following the stanard protocol
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Quickchange of the EaglII restriction site in the PMA_RepCap vector</b></p>====
| + | |
| - | Investigator: <b>Chris L., Achim</B>
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: We performed a QuikChange mutagenesis on the synthesized PMA_RepCap vector (P190). The EaglII restriction enzyme also cuts NotI (IGEM standard) therefore EaglII has to be changed into a different recognition site. Primers were designed to change it into PvuII. The new QuikChange Lighting Site-Directed Mutagenesis Kit from Stratagene was used, therefore the mutagenesis was carried out as described in the QuikChange Manual. The PCR product was transformed into XL10-Gold cells, we used the IGEM standard protocol, except for the addition of ß-ME to the XL10-Gold cells. : </p>
| + | |
| - | | + | |
| - | PCR-reaction:
| + | |
| - | {| border="1"
| + | |
| - | | '''Volume / µl''' || align="right" |'''ingredients'''|| align="right" |'''recommended /µl'''
| + | |
| - | |-
| + | |
| - | | 2,5 || align="right" |10X reaction buffer || align="right" | 2,5
| + | |
| - | |-
| + | |
| - | | 2,9 || align="right" |P190 template (~10 ng)|| align="right" | 2,9 of 1:100 dilution
| + | |
| - | |-
| + | |
| - | | 0,51|| align="right" |forward primer: O122|| align="right" | 62,5 ng
| + | |
| - | |-
| + | |
| - | |0,51 || align="right" |reverse primer: O123 || align="right" | 62,5 ng
| + | |
| - | |-
| + | |
| - | |- || align="right" |DMSO || align="right" | -
| + | |
| - | |-
| + | |
| - | |0,5|| align="right" |dNTP|| align="right" | 250 µM each dNTP
| + | |
| - | |-
| + | |
| - | |17,6|| align="right" |H<sub>2</sub>O || align="right" |
| + | |
| - | |-
| + | |
| - | |0,5|| align="right" |QuikChange Lightning ennzyme(1.25) || align="right" |
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| - | PCR program:
| + | <li>1% agarose gel was repared, gel ran for 45 minutes(119 V) |
| - | {| border="1"
| + | |
| - | |'''Rounds'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time'''
| + | |
| - | |-
| + | |
| - | |1 || align="right" |95 || align="right" | 2 minutes
| + | |
| - | |-
| + | |
| - | |18 || align="right" |95|| align="right" |20 seconds
| + | |
| - | |-
| + | |
| - | |18 || align="right" |60 || align="right" | 10 seconds
| + | |
| - | |-
| + | |
| - | |18|| align="right" |68|| align="right" | 2 minutes (4 kb * 30 sec)
| + | |
| - | |-
| + | |
| - | |1|| align="right" |68|| align="right" | 5 minutes
| + | |
| - | |}
| + | |
| - | | + | |
| | <br> | | <br> |
| - | The PCR product was digestet with Dpn I for 10 minutes at 37°C
| + | Results: Test digestion of pCMV_MCS with NotI delivered plausibel results (expected fragment size: ~ 27 kbp and 17 kbp). |
| - | | + | Unfortunately the digestion of pCMV_mVenus_YFP with PstI and MluI resulted in one 2.9 kbp and one 1.2 kbp fragment, which didn't correspond to the expected fragment sizes of 19 kbp and 33 kbp - but to fragment sizes of pCMV_MCS without mVenus_YFP! |
| - | Plasmid was transformed into XL10-Gold cells.
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#ff00ff;"><b>Sequencing results of Rep68, Rep78 and AAP BioBricks</b></p>====
| + | |
| - | Investigator: <b>Hanna</B>
| + | |
| - | <br />
| + | |
| - | Comment: pSB1C3_Rep78 (P160), pSB1C3_Rep68 (P165) and pSB1C3_AAP (P170) were sent for sequencing twice. No sequencing made sense. Paradoxically the Rep78 sequencing result fit to the p5 promoter BioBrick... <br/>
| + | |
| - | By having a closer look to the BioBrick PCR we found out that the fragment sizes didn't fit to the expected sequences. In addition to that the test digestion also showed some discrepancies. <br/>
| + | |
| - | Because we weren't able to find out in which steps of the BioBrick production things went wrong, we decided to discard all referring glycerol stocks and plasmids and to perform the Rep68, Rep78 and AAP BioBrick PCR again!!!
| + | |
| - | <br/>
| + | |
| - | <br/>
| + | |
| - | ====<p style="font-size:15px; background-color:#9acd32;"><b>Comment 13.08.2010 (Hanna):</b> We wondered how the whole Rep stuff could be sequenced reverse AND forward without any forward sequencing primer... By checking the "sequencing book" we found out that always the wrong primers were used: the GOI primers can be used in order to sequence the <b>G</b>ene <b>o</b>f <b>i</b>nterest = GOI (!) in pAAV_MCS and NOT the pSB1C3!!!!!!!!!</p>====
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Order oligos for CD-biobrick</b></p>====
| + | |
| - | '''Investigator: Jessica and Kira'''
| + | |
| - | <br>ordered on aug 12th<br>
| + | |
| - | [[File:Freiburg10 oligos for CD-biobrick.jpg]]
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>BioBrick assembly of pSB1C3_leftITR_CMV_mVenus</b></p>====
| + | |
| - | | + | |
| - | Investigator: Stefan <br />
| + | |
| - | | + | |
| - | comment: In order to assemble our complete constructs mVenus has to be included into the pSB1C3_leftITR_CMV vector. <br />
| + | |
| - | <b>Digestion:</b> <br />
| + | |
| - | {| border="1"
| + | |
| - | | components: || align="right" |pSB1C3_leftITR_CMV P188(Vector) || align="right" |pGA14_mVenus P60 (Insert)
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |6 || align="right" |8
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |2 || align="right" | 2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |2 || align="right" |2
| + | |
| - | |-
| + | |
| - | |Enzyme1 || align="right" |1 (SpeI) || align="right" |1 (Xba1)
| + | |
| - | |-
| + | |
| - | |Enzyme2 || align="right" |1 (PstI)|| align="right" |1 (PstI)
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |8 || align="right" |6
| + | |
| - | |-
| + | |
| - | |'''Total volume'''|| align="right" | 20|| align="right" |20
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | | + | |
| - | | + | |
| - | Preparation of gel:<br />
| + | |
| - | 0,5 g Agarose,50 ml TAE (1%), 3 µl GELRED , at 110 Volt, running time: 45 minutes
| + | |
| - | <br />
| + | |
| - | | + | |
| - | | + | |
| - | [[File:Freiburg10 cloning of pSB1C3 leftITR CMV mVenus.png|500px]] <br />
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | Expected sizes of constructs:
| + | |
| - | *pSB1C3_leftITR_CMV: 2869 bp
| + | |
| - | *pGA14_mVenus: 2896 bp, 756 bp
| + | |
| - | | + | |
| - | The corresponding bands were cut out and Gel-Extraction was performed according to protocol.
| + | |
| - | <br />
| + | |
| - | | + | |
| - | concentrations measured via NanoDrop:
| + | |
| - | *pSB1C3_leftITR_CMV: 39,2 ng/µl
| + | |
| - | *pGA14_mVenus: 8,2 ng/µl
| + | |
| - | | + | |
| - | | + | |
| - | <b>Ligation:</b> <br />
| + | |
| - | | + | |
| - | T4 ligation was performed according to protocol. <br />
| + | |
| - | | + | |
| - | Volumes used:
| + | |
| - | *vector: 1,67 ng/µl
| + | |
| - | *insert: 6,33 ng/µl
| + | |
| - | | + | |
| - | | + | |
| - | <b>Transformation:</b> <br />
| + | |
| - | | + | |
| - | bacterial strain used: XL1bB<br />
| + | |
| - | | + | |
| - | antibiotic used for plate: chlorampenicol<br />
| + | |
| - | | + | |
| - | Transformation was performed according to protocol.<br />
| + | |
| - | Plate was prepared and put in 37°C room.
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Opened a BioBrick box</b></p>====
| + | |
| - | <b>Investigator: Bea</b>
| + | |
| - | <br />
| + | |
| - | In order to "save" the plasmids/BioBricks which has been prepared and confirmed for submitting it to the parts registry a new box have been prepared. The box should contain 30 µL of the desired construct cloned into the pSB1C3 vector and the BBa number given from the headquarter. <br />
| + | |
| - | | + | |
| - | [[File:Freiburg10 BioBrick highlighting in Nomenclature list.JPG|400px|thumb]]
| + | |
| - | The first BioBricks which are in this box are:
| + | |
| - | <ul>
| + | |
| - | <li>pSB1C3_hGH (P136): Bba_K404002</li>
| + | |
| - | <li>pSB1C3_betaglobin (P133: Bba_K404001 </li>
| + | |
| - | <li>pSB1C3_CMV (P145): Bba_K404003</li>
| + | |
| - | <li>pSB1C3_leftITR (P175): Bba_K404006</li>
| + | |
| - | <li>pSB1C3_rightITR (P172): Bba_K404005</li>
| + | |
| - | <li>pSB1C3_mGMK (P156): Bba_K404004</li>
| + | |
| - | </ul>
| + | |
| - | In our nomenclature plasmid excel list the constructs which we aliquoted should be highlighted in blue and commented with the given Bba number. Additionally it should be taken in consideration that enough DNA should be in the "working" plasmid box. Therefore, if necessary, inoculate DYT medium for Mini-Preps.<br />
| + | |
| - | <br />
| + | |
| - | In this case ALL the glycerol stocks were used for incoluating 10 mL DYT medium containing 10 µL chloramphenicol respectively.
| + | |
| - | <ul>
| + | |
| - | <p style="color:#66bbff;">To do: Mini-Preps of the over night cultures.</p>
| + | |
| - | </ul>
| + | |
| - | <br/>
| + | |
| - | | + | |
| - | === 88. labday 13.08.2010===
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Continuation:pSB1C3_SDM_SspI_bla14FM</b></p>====
| + | |
| - | Investigator: Patrick <br>
| + | |
| - | Intention: receive enough plasmid for a sequencing and maybe futher working steps. <br>
| + | |
| - | Three clones were picked in the morning to inoculate DYT and perform a mini-prep in the evening and to send them for sequencing. The three clones were picked and prepped according to the standard protocol yielding the following DNA concentrations:
| + | |
| - | *pSB1C3_SDM_SspI_bla14FM clone 1: 65,15 ng/µl , Sequencing labeled: PS3.1
| + | |
| - | *pSB1C3_SDM_SspI_bla14FM clone 2: 22,3 ng/µl , Sequencing labeled: PS3.2
| + | |
| - | *pSB1C3_SDM_SspI_bla14FM clone 3: 62,3 ng/µl , Sequencing labeled: PS3.3
| + | |
| | <br> | | <br> |
| - | Used primer: seq_pSB1C3_VR2_rev (O51) <br>
| |
| - | Results: see http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/index.php/Laborjournal#Sequence_analysis_of_pSB1C3_SDM_SspI_BLA
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-preps of pSB1C3_hgh,pSB1C3_beta globin,pSB1C3_left ITR,pSB1C3_right ITR,pSB1C3_mGMK,pSB1C3_CMV</b></p>====
| |
| - | <br/>
| |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>:new mini-preps of the glycerol-stocks ofpSB1C3_hgh(=B103),pSB1C3_beta globin(=B100),pSB1C3_left ITR (=B143),pSB1C3_right ITR (=B140),pSB1C3_mGMK (=B125),pSB1C3_CMV : </p>
| |
| - | <br/>
| |
| - | {| border="1"
| |
| - | |'''components'''|| align="right" |'''concentration in ng/µl'''
| |
| - | |-
| |
| - | | P191 (pSB1C3_right ITR)|| align="right" | 100,74
| |
| - | |-
| |
| - | |P192 (pSB1C3_beta-globin) || align="right" |240,08
| |
| - | |-
| |
| - | |P193 (pSB1C3_CMV) || align="right" | 333,89
| |
| - | |-
| |
| - | |P194 (pSB1C3_hgh) || align="right" |328,42
| |
| - | |-
| |
| - | | P195 (pSB1C3_mGMK) || align="right" |258,63
| |
| - | |-
| |
| - | | P196 (pSB1C3_left ITR) || align="right" |173,98
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#FF00ff;"><b>Design and ordering of oligos for VP fusion targeting approaches</b></p>====
| |
| - | '''Investigator: Hanna'''
| |
| - | <br/>
| |
| - | <p> <b>Comment:</b> In order to target the EGFR receptor of our A431 cells different approaches have been figured out: Besides loop insertions, N-terminal fusion to the VP2 protein will be performed. In addition to that a VP1 insertion approach (after Grieger et al., 2007) was planned. <br/>
| |
| - | Fusion and insertion molecules will be e.g. His-tag, Affibody, Darpin, GFP. These approaches can be combinated with the loop insertions. <br/>
| |
| - | For this purpose the following oligos were ordered today: <br/></p>
| |
| - | <br/>
| |
| - | [[File:Freiburg10 N-terminalFusion1.jpg|450px|thumb|left|]]
| |
| - | [[File:Freiburg10 N-terminalFusion2.jpg|450px|thumb|right|]]
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - | <br/>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep and Test digestion of Rep40 and Rep52 of the cloning experiment ("Cloning of Rep40/52" 11.08.2010 Investigator: Anna) </b></p>====
| |
| - | Investigator: <b>Chris L., Achim</B>
| |
| - | <br />
| |
| - |
| |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: There are small and big colonies on the plate. Maybe problems with the agar plate. Picked colonies of pSB1C3_Rep40 sample 1 and pSB1C3_Rep52 sample 1 are small colonies. Picked colonies of pSB1C3_Rep40 sample 2 + 3 and pSB1C3_Rep52 sample 2 + 3 are standard size colonies.</p>
| |
| - | <br/>
| |
| - |
| |
| - | '''Nanodrop'''
| |
| - | * Rep 40 Sample 1: 156,26 ng/µl '''P197'''
| |
| - | * Rep 40 Sample 2: 200,05 ng/µl '''P198'''
| |
| - | * Rep 40 Sample 3: 144,06 ng/µl '''P199'''
| |
| - | * Rep 52 Sample 1: 173,36 ng/µl '''P200'''
| |
| - | * Rep 52 Sample 2: 160,75 ng/µl '''P201'''
| |
| - | * Rep 52 Sample 3: 295,77 ng/µl '''P202'''
| |
| - |
| |
| | <br> | | <br> |
| | <br> | | <br> |
| - | '''Test digestion''' 800ng DNA | + | <li>'''Ordering oligos:'''(Sigma-Aldrich) <br> |
| - | {| border="1"
| + | (Bea)<br> |
| - | | components || align="right" |P145 /µl || align="right" |P146 /µl || align="right" |P145 /µl || align="right" |P146 /µl|| align="right" |P145 /µl || align="right" |P146 /µl
| + | Oligos have been ordered for modifying the ITRs. Deletion of PstI restriction site in ITR. <br> |
| - | |-
| + | |
| - | | DNA || align="right" | 5,1|| align="right" |4|| align="right" |5,6|| align="right" |4,6|| align="right" |5|| align="right" |2,7
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" | 2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" | 2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2
| + | |
| - | |-
| + | |
| - | | Enzyme 1: XbaI || align="right" | 0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5
| + | |
| - | |-
| + | |
| - | | Enzyme 2: SpeI || align="right" | 0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5
| + | |
| - | |-
| + | |
| - | |H<sub>2</sub>O|| align="right" | 9,9|| align="right" |11|| align="right" |9,4|| align="right" |10,4|| align="right" |10|| align="right" |12,3
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''
| + | |
| - | |}
| + | |
| - | <br> | + | |
| - | Incubation time: 45 minutes, Incubation temperature: 37°
| + | |
| - | <br> | + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | 0,5 g Agarose, 50ml x TAE, 3µl GELRED, at 115 Volt, running time: 45 minutes
| + | |
| | | | |
| - |
| |
| - | {| border="1" cellspacing="0" cellpadding="2" bordercolor="black"
| |
| - | !Sample
| |
| - | !Sample/µl]
| |
| - | !Loading dye (6x)/µl
| |
| - | !Expected size 1 (Geneious)
| |
| - | !Expected size 2 (Geneious)
| |
| - | |--
| |
| - | |Rep 40 1
| |
| - | |20 µl
| |
| - | | 4 µl
| |
| - | |____ bp
| |
| - | | 940 bp
| |
| - | |--
| |
| - | |Rep 40 2
| |
| - | |20 µl
| |
| - | | 4 µl
| |
| - | |____ bp
| |
| - | | 940 bp
| |
| - | |--
| |
| - | |Rep 40 3
| |
| - | |20 µl
| |
| - | | 4 µl
| |
| - | |____ bp
| |
| - | | 940 bp
| |
| - | |--
| |
| - | |Rep 52 1
| |
| - | |20 µl
| |
| - | | 4 µl
| |
| - | |____ bp
| |
| - | |1198 bp
| |
| - | |--
| |
| - | |Rep 52 2
| |
| - | |20 µl
| |
| - | | 4 µl
| |
| - | |____ bp
| |
| - | |1198 bp
| |
| - | |--
| |
| - | |Rep 52 3
| |
| - | |20 µl
| |
| - | | 4 µl
| |
| - | |____ bp
| |
| - | |1198 bp
| |
| - | |--
| |
| - | |}
| |
| - | {| align=right
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | {| border="1"
| |
| - | |
| |
| - | !Marker
| |
| - | !Sample Rep40 1 /20µl
| |
| - | !Sample Rep40 2 /20µl
| |
| - | !Sample Rep40 3 /20µl
| |
| - | !Sample Rep52 1 /20µl
| |
| - | !Sample Rep52 2 /20µl
| |
| - | !Sample Rep52 3 /20µl
| |
| - | |-
| |
| - | !Lane
| |
| - | | 1
| |
| - | | 2
| |
| - | | 3
| |
| - | | 4
| |
| - | | 5
| |
| - | | 6
| |
| - | | 7
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - | agarose gel: 1%, digestion: 45 minutes, 37°C
| |
| - |
| |
| - |
| |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments:'''''Results look bad. Rep52 clone 3 looks good, send for sequencing. </p><br>
| |
| - | '''</p>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Repetition of Rep 40 and Rep 52 ligation and transformation with the vector pSB1C3_CFP</b></p>====
| |
| - |
| |
| - | Investigator: <b>Chris L.</B>
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - | *Ligation of PCR products and vector:
| |
| - |
| |
| - | For the Ligation 1µl T4 buffer (10x) and 1µl T4 ligase were used.
| |
| - | <br />
| |
| - | {| border="1"
| |
| - | | ''' ''' || align="right" |'''Sample-no.'''|| align="right" |'''vector /µl''' || align="right" |'''insert /µl'''
| |
| - | |-
| |
| - | | pSB1C3 + Rep40 || align="right" |1 || align="right" |4,92 || align="right" |3,08
| |
| - | |-
| |
| - | | pSB1C3 + Rep52 || align="right" |2 || align="right" |6,47 || align="right" |1,53
| |
| - | |-
| |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | || align="right" | 10 || align="right" | 10
| |
| - | |}
| |
| - | <br />
| |
| - |
| |
| - | *Transformation:
| |
| - |
| |
| - | The transformation was done following the standard protocol using XL1B cells.
| |
| - | <br/>
| |
| - | <br/>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#ff00ff;"><b>Strategy for VP2 N-terminal fusion and VP1 insertion of targeting moelcules</b></p>====
| |
| - |
| |
| - | Investigator: <b>Hanna</B>
| |
| - | <br />
| |
| - | <p><b>Comment:</b> In order to target the EGFR receptor of tumor cells via Affibody or Darpin (antibody fragment is also possible!!!), or in order to expose e.g. His-Tags on the virus surface, two approaches have been figured out. <br/>
| |
| - | <br/>
| |
| - | [[File:Freiburg10_N-terminalFusion_Strategy.JPG]]
| |
| - | <br/>
| |
| - | [[File:Freiburg10_N-terminalFusion_Strategy2.JPG]]
| |
| - | [[File:Freiburg10 N-terminalFusion Strategy3.JPG]]
| |
| - | <br/>
| |
| - |
| |
| - |
| |
| - | ===='''pSB1C3_Rep52 (P202) sent for sequencing'''====
| |
| - |
| |
| - | '''Investigator: Chris L.'''<br>
| |
| - |
| |
| - | Primer used: Reverse Primer VR2
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;">'''Repetition: Biobrick production of Rep 68, 78 and AAP'''</p>====
| |
| - |
| |
| - | '''Investigator: Stefan'''<br>
| |
| - |
| |
| - |
| |
| - | Aim of the experiment:
| |
| - | We want to produce biobricks from Rep 68, Rep 78 and the AAP.
| |
| - |
| |
| - | *Plasmids used as template:
| |
| - | Rep_68_ex (p119): c = 470,6 ng/µl <br>
| |
| - | Rep_78_(p122): c = 566,8 ng/µl <br>
| |
| - | pAAV-RC containing AAP ORF (p50): c = 378,5 ng/µl <br>
| |
| - |
| |
| - |
| |
| - |
| |
| - | *Primer used:
| |
| - | For Rep_68_ex: Praefix_68_78_ex & Suffix_40_68_ex<br>
| |
| - | For Rep_78_ex: Praefix_68_78_ex & Suffix_52_78_ex<br>
| |
| - | For AAP_ex: Praefix_AAP_ex & Suffix_AAP_ex
| |
| - |
| |
| - | <br>
| |
| - | *'''PCR:
| |
| - | (was performed following the standard protocol)
| |
| - | <br>
| |
| - | {| border="1"
| |
| - | | '''Ingredients''' || align="right" |'''Volume / µl''' || align="right" |'''Rep68'''|| align="right" |'''Rep78''' || align="right" |'''AAP'''
| |
| - | |-
| |
| - | | 5X Phusion HF buffer || align="right" |10 || align="right" | || align="right" | || align="right" |
| |
| - | |-
| |
| - | | 10 mM dNTP mix|| align="right" |1|| align="right" | || align="right" | || align="right" |
| |
| - | |-
| |
| - | | forward primer: || align="right" |2,5|| align="right" | || align="right" | || align="right" |
| |
| - | |-
| |
| - | | reverse primer: || align="right" |2,5|| align="right" | || align="right" | || align="right" |
| |
| - | |-
| |
| - | | DNA Template|| align="right" |***|| align="right" |2,5 µl || align="right" |2 µl|| align="right" |3 µl
| |
| - | |-
| |
| - | | DMSO (2%)|| align="right" | || align="right" | - || align="right" | - || align="right" | 1 µl
| |
| - | |-
| |
| - | | Phusion Polymerase|| align="right" |0,5|| align="right" | || align="right" | || align="right" |
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| align="right" |*** || align="right" | 31 µl|| align="right" | 31,5 µl|| align="right" |29,5 µl
| |
| - | |-
| |
| - | |Total volume|| align="right" |50
| |
| - | |}
| |
| | <br> | | <br> |
| | + | '''right ITR of pAAV_MCS''' <br> |
| | | | |
| - | PCR program:
| + | oligos ordered:<br> |
| - | {| border="1"
| + | |
| - | |'''PCR Program'''|| align="right" |'''temperature/ °C'''|| align="right" |'''Time''' || align="right" |'''Rep68'''|| align="right" |'''Rep78'''|| align="right" |'''AAP'''
| + | fwd: 5´- gcgcagctgcctgcaCGGGCGCCTGATGCGG -3´ 77 °C, 31 bp <br> |
| - | |-
| + | rev: 5´- CCGCATCAGGCGCCCGTGCAGGCAGCTGCGC -3´ <br> |
| - | |1|| align="right" |98 || align="right" |1min|| align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |2|| align="right" |98 || align="right" |15s|| align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |3|| align="right" |*** || align="right" |25s|| align="right" |63°C|| align="right" |62°C|| align="right" |64°C
| + | |
| - | |-
| + | |
| - | |4 (step 2-4 8x) || align="right" |72|| align="right" |***|| align="right" |24s || align="right" |27s || align="right" | 10s
| + | |
| - | |-
| + | |
| - | |5 || align="right" |98|| align="right" | 15s|| align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |6|| align="right" |***|| align="right" |25s|| align="right" |68°C|| align="right" |64°C|| align="right" |68°C
| + | |
| - | |-
| + | |
| - | |7|| align="right" |72|| align="right" |***|| align="right" |24s|| align="right" |27s|| align="right" |10s
| + | |
| - | |-
| + | |
| - | |8 (step 6-8 17x)|| align="right" |72|| align="right" |5min|| align="right" | || align="right" | || align="right" |
| + | |
| - | |-
| + | |
| - | |Hold|| align="right" |4|| align="right" | || align="right" | || align="right" | || align="right" |
| + | |
| - | |}
| + | |
| | <br> | | <br> |
| | | | |
| - | Used agarose gel:
| + | '''leftITR of pAAV_MCS'''<br> |
| - | 0,5 g Agarose,50 ml TBE (1%), 3 µl GELRED , at 115 Volt, running time:45 minutes
| + | |
| | | | |
| - | [[File:Freiburg10 68 78 AAP.jpg|500px|]]
| + | oligos ordered:<br> |
| | + | |
| | + | fwd: 5´- CCTTTTGCTCACATGTCGTGCAGGCAGCTGCGCG -3´ 74 °C, 34 bp <br> |
| | + | rev: 5´- CGCGCAGCTGCCTGCACGACATGTGAGCAAAAGG -3´<br> |
| | + | <br> |
| | + | For further details see link <br> |
| | | | |
| - | | + | http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/images/4/44/Freiburg10_Oligos_ITR_mutagenesis_for_pAAV_delete_PstI.pdf |
| - | <p style="font-size:15px; font-weight: bold; color: blue;">Gel extraction</p>
| + | </li> |
| - | <br />
| + | |
| - | Gel extraction was perfomed according to protocol.
| + | |
| - | | + | |
| - | *Digestion of PCR products and vector:
| + | |
| - | | + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | '''components''' || align="right" |'''PCR products /µl''' || align="right" |'''vector /µl'''
| + | |
| - | |-
| + | |
| - | | DNA || align="right" |29 || align="right" |11
| + | |
| - | |-
| + | |
| - | | BSA (10x) || align="right" |4 || align="right" | 2
| + | |
| - | |-
| + | |
| - | | Buffer 4 (10x)|| align="right" |4 || align="right" |2
| + | |
| - | |-
| + | |
| - | |Enzyme '''XbaI || align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |Enzyme '''SpeI || align="right" |1 || align="right" |1
| + | |
| - | |-
| + | |
| - | |H2O|| align="right" |1 || align="right" |3
| + | |
| - | |-
| + | |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | 40|| align="right" |20
| + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#68bbff;">'''''Comments''''': Digestion was done with XbaI and SpeI, it has to be checked if the inserts are cloned into the vector in the right orientation. </p> <br>
| + | |
| - | | + | |
| - | *Purification of Rep68, 78 and AAP:
| + | |
| - | For the purification 200 µl of buffer PBI was used.
| + | |
| - | | + | |
| - | c(Rep68)= 14,3 ng/µl <br />
| + | |
| - | c(Rep78)= 12,0 ng/µl <br />
| + | |
| - | c(AAP)= 25,1 ng/µl
| + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | *Gelextraction of pSB1C3_RFC25_CFP:
| + | |
| - | 0,5 g Agarose,50 ml TBE (1%), 3 µl GELRED , at 115 Volt, running time:50 minutes <br />
| + | |
| - | 4µl loading dye (6x) for the sample, Marker: GeneRuler ladder mix (Fermentas)
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | [[File:Freiburg10 pSB1C3 CFP 130810.jpg|500px|]]
| + | |
| - | | + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | | + | |
| - | c(pSB1C3)= 6,9 ng/µl <br />
| + | |
| - | | + | |
| - | | + | |
| - | <br />
| + | |
| - | | + | |
| - | *T4 ligation of PCR products and vector:
| + | |
| - | | + | |
| - | For the Ligation 1µl buffer (10x) and 1µl T4 ligase were used.
| + | |
| - | <br />
| + | |
| - | {| border="1"
| + | |
| - | | ''' ''' || align="right" |'''vector /µl''' || align="right" |'''insert /µl'''
| + | |
| - | |-
| + | |
| - | | pSB1C3 + Rep68 || align="right" |3,78 || align="right" |4,24
| + | |
| - | |-
| + | |
| - | | pSB1C32 + Rep78 || align="right" |3,13 || align="right" |4,87
| + | |
| - | |-
| + | |
| - | | pSB1C3 + AAP || align="right" |6,42 || align="right" |1,58
| + | |
| - | |-
| + | |
| - | | + | |
| - | |}
| + | |
| - | <br />
| + | |
| - | | + | |
| - | *Transformation:
| + | |
| - | | + | |
| - | The transformation was done following the standard protocol using XL1b cells.
| + | |
| - | | + | |
| - | === 89. labday 14.08.2010===
| + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Sequence analysis of pSB1C3_SDM_SspI_BLA</b></p>====
| + | |
| - | Investigator: <b>Patrick, Bea </b>
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: Sequence analysis of three clones revealed that all sequences contain some mutations in the regions where the primer annealed. This was due to the extreme primer lentgh. Primers that long should be ordered as HPLC purified in order to avoid mutations in the primer sequence.</p>
| + | |
| - | <ol>
| + | |
| - | <li>Clone 1: Mutation in the SalI site of the loop insertion site in the Viral Brick MCS lead to the removal of the recognition site. CANNOT be used for the loop insertions!!!!</li>
| + | |
| - | <li>Clone 2: Mutation in the region of the primer upstream of the loop insertion sites SalI and PvuII.</li>
| + | |
| - | <li>Clone 3: Mutation in the region of the primer downstream of the loop insertion sites SalI and PvuII.</li>
| + | |
| - | </ol>
| + | |
| - | <br />
| + | |
| - | <gallery widths=450px heights=300px perrow=2 caption="Sequencing results of pSB1C3_SDM of SspI_BLA clone 2 and pSB1C3_SDM of SspI_BLA clone 3">
| + | |
| - | Image:Freiburg10 Sequence analysis of pSB1C3 SDM SspI BLA clone2.jpg|Clone 2
| + | |
| - | Image:Freiburg10 Sequence analysis of pSB1C3 SDM SspI BLA clone3.jpg|Clone 3
| + | |
| - | </gallery>
| + | |
| - | <br />
| + | |
| - | <p style="font-size:13px; color:#33cc66;"><b>Next steps: </b> Two additional clones will be picked and sent for sequencing (clones 4 and 5). In order to obtain a construct without any mutations, clone 2 and clone 3 couldbe cloned together. Each clone will be digested with SalI and BamHI. Clone 2 will be the "insert", clone 3 is going to be the "vector". Therefore the fragments will (hopefully) not contain anymore mutations in the relevant sequence. </p>
| + | |
| - | | + | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini Preps of PMA_RepCap vector and pSB1C3_left ITR_CMV_betaglobin_mVenus</b></p>====
| + | |
| - | <br/>
| + | |
| - | '''Investigator: Chris L., Bea'''
| + | |
| | <br> | | <br> |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>:new mini-preps of the glycerol-stocks of pSB1C3_leftITR_CMV_betaglobin_mVenus clone 1 (=B179), pSB1C3_leftITR_CMV_betaglobin_mVenus clone 2 (=B180), pSB1C3_leftITR_CMV_betaglobin_mVenus clone 3 (=B181), pMA_RepCap Vector_SDM_InsPvuII clone 1, (=B182), pMA_RepCap Vector_SDM_InsPvuII clone 2 (=B183) and pMA_RepCap Vector_SDM_InsPvuII clone 3 (=B184)</p>
| |
| - | <br/>
| |
| - | <b>Comment: The plamsid numbers in the picture (P179-P184) correspond to the glycerol stocks B179-B184. The corresponding plasmid numbers are: P208-P213. </b>
| |
| - | <br />
| |
| - | '''Nanodrop'''
| |
| - | * pSB1C3_leftITR_CMV_betaglobin_mVenus clone 1: 256,64 ng/µl '''B179'''
| |
| - | * pSB1C3_leftITR_CMV_betaglobin_mVenus clone 2: 274,86 ng/µl '''B180'''
| |
| - | * pSB1C3_leftITR_CMV_betaglobin_mVenus clone 3: 217,20 ng/µl '''B181'''
| |
| - | * pMA_RepCap Vector_SDM_InsPvuII clone 1: 253,71 ng/µl '''B182'''
| |
| - | * pMA_RepCap Vector_SDM_InsPvuII clone 2: 195,64 ng/µl '''B183'''
| |
| - | * pMA_RepCap Vector_SDM_InsPvuII clone 3: 144,61 ng/µl '''B184'''
| |
| | | | |
| | + | '''Test-Trafo of chemical competent E.coli cells''' <br> |
| | + | (Hanna) |
| | <br> | | <br> |
| | + | The competent E.coli XL-1-blue and competent E.coli BL21 which were prepared the day before were test-transformed with pUC18 - following the standard protocol. Cells were plated on agar plates containing ampicillin and stored over night at 37°C. |
| | + | Further on two control plates containing ampicillin or kanamycin were prepared. Non-transformed cells were plated on them and also stored over night at 37°C. |
| | <br> | | <br> |
| - | '''Test digestion''' 800ng DNA
| |
| - | {| border="1"
| |
| - | | components || align="right" |B179 /µl || align="right" |B180 /µl || align="right" |B181 /µl || align="right" |B182 /µl|| align="right" |B183 /µl || align="right" |B184 /µl|| align="right" |pMA_RepCap Vector /µl
| |
| - | |-
| |
| - | | DNA || align="right" | 3,1|| align="right" |2,9|| align="right" |3,7|| align="right" |3,1|| align="right" |4|| align="right" |5,5|| align="right" | 2,4
| |
| - | |-
| |
| - | | BSA (10x) || align="right" | 2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2
| |
| - | |-
| |
| - | | Buffer 4 (10x)|| align="right" | 2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2|| align="right" |2
| |
| - | |-
| |
| - | | Enzyme 1: NgoMIV || align="right" | 0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0|| align="right" |0|| align="right" |0 || align="right" |0
| |
| - | |-
| |
| - | | Enzyme 2: EcoRI || align="right" | 0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0|| align="right" |0|| align="right" |0|| align="right" |0
| |
| - | |-
| |
| - | | Enzyme 2: PvuII || align="right" | 0|| align="right" |0|| align="right" |0 || align="right" |0,5|| align="right" |0,5|| align="right" |0,5|| align="right" |0,5
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| align="right" | 11,9|| align="right" |12,1|| align="right" |11,3|| align="right" |12,4|| align="right" |11,5|| align="right" |10|| align="right" | 13,1
| |
| - | |-
| |
| - | |'''Total volume (e.g. 15,20,25,30 µl)'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''|| align="right" | '''20'''
| |
| - | |}
| |
| | <br> | | <br> |
| - | Incubation time: 45 minutes, Incubation temperature: 37°
| + | </ul> |
| - | <br>
| + | </ul> |
| - | <br> | + | ===16. Labortag 27.05.2010=== |
| - | <br> | + | |
| - | 0,5 g Agarose, 50ml x TAE, 3µl GELRED, at 115 Volt, running time: 45 minutes
| + | |
| | | | |
| - | <br /> | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>Cell counting</b></p>==== |
| - | <b>Results of test digestion</b> | + | |
| | | | |
| - | [[File:Freiburg10 Test digestion of pSB1C3 Bba fourparts SDM pMA RC insert 14 08.jpg|500px]]
| + | <p><b>Investigator: Patrick, Adrian, Chris W. Christian L.</b></p> |
| - | <br /> | + | |
| - | <br />
| + | |
| - | <br />
| + | |
| - | <b>Results</b>: Two (three) different approaches can be seen on the agarose gel. | + | |
| - | <ol>
| + | |
| - | <li style="font-size:13px; color:#66bbff;">P178-P181 (blue): The plasmid pSB1C3_leftITR_CMV_betaglobin_mVenus was digested with NgoMIV and EcoRI. Expected bands were: <b> 2873bp and 1343 </b>. As it can be seen in the picture, the two fragments ran too high (~300bp). As it is almost normal for the pSB1C3 plasmid, we should figure out why this is always the problem for this vector.</li>
| + | |
| - | <li style="font-size:13px; color:#cc3333;">P182-P183 (red): The plasmid pMA_RepCap_SDM_insPvuII was digested with PvuII as well as the control vetor pMA_RepCap where no site-directed mutagenesis was performed in order to see the difference. As it can be seen in the picture above, the expected band sizes of <b>263bp,1384bp and 2133bp</b> correspond to the bands detected in the gel. The control vector instead reveals only two bands detectable. Therefore site-directed mutagenesis was successfully performed.</li>
| + | |
| - | <li style="font-size:13px; color:#33cc33;">P177 (green): pSB1C3_hTERT. For further details see extra part below (Test digestion of pSB1C3_hTERT.</li>
| + | |
| - | </ol> | + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep of pSB1C3_leftITR_CMV_mVenus</b></p>====
| + | * Meeting |
| - | <b>Investigator: Stefan</b><br>
| + | |
| | | | |
| - | Glycerol stocks were prepared:
| + | cell counting: |
| - | <li>B177 = pSB1C3_leftITR_CMV_mVenus clone 1
| + | |
| - | <li>B178 = pSB1C3_leftITR_CMV_mVenus clone 2
| + | |
| - | | + | |
| - | | + | |
| - | Mini-Prep following the standard protokol
| + | |
| - | <br />
| + | |
| - | <li>P206 = pSB1C3_leftITR_CMV_mVenus clone 1 = 277,9 ng/µl
| + | |
| - | <li>P207 = pSB1C3_leftITR_CMV_mVenus clone 2 = 260,3 ng/µl
| + | |
| - | | + | |
| - | <br />
| + | |
| | <br> | | <br> |
| | + | * BL21 + PUC18 Trafo 54*4 = 216 |
| | + | * XL1B + PUC18 Trafo 30*4 = 120 |
| | + | digitalization of recipe-cards (Christian L.) |
| | <br> | | <br> |
| - | <b>Test digestion:</b>
| |
| - | or test digestion, pSB1C3_leftITR_CMV (P188) was digested as well. <br />
| |
| - |
| |
| - | {| border="1"
| |
| - | | '''Ingredients''' || align="right" |'''clone 1/µl'''|| align="right" |'''clone 2/µl''' || align="right" |'''P188/µl'''
| |
| - | |-
| |
| - | | DNA: || align="right" |3,5 || align="right" |3,5 || align="right" |4
| |
| - | |-
| |
| - | | BSA (10x): || align="right" |2 || align="right" |2 || align="right" |2
| |
| - | |-
| |
| - | | Buffer 4: || align="right" |2 || align="right" |2 || align="right" |2
| |
| - | |-
| |
| - | | Enzyme 1 EcoRI: || align="right" |0,5 || align="right" |0,5 || align="right" |0,5
| |
| - | |-
| |
| - | | Enzyme 2 AgeI|| align="right" |0,5 || align="right" |0,5|| align="right" |0,5
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| align="right" | 6,5|| align="right" | 6,5|| align="right" |6
| |
| - | |-
| |
| - |
| |
| - | |}
| |
| - |
| |
| - | <br />
| |
| - | <ul>
| |
| - | All samples were loaded on a 1% agarose gel:
| |
| - | <li>GeneRuler Ladder Mix (7µl)</li>
| |
| - | <li>P206 = pSB1C3_leftITR_CMV_mVenus clone 1 </li>
| |
| - | <li>P207 = pSB1C3_leftITR_CMV_mVenus clone 2 </li>
| |
| - | <li>P188 = pSB1C3_leftITR_CMV</li>
| |
| - |
| |
| - | </ul>
| |
| - | <br />
| |
| - | [[File:Freiburg10 test digestion leftITR CMV mVenus 140810.jpg|300px]]
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | comment: Through digestion, fragments would have similar sizes. Therefore, a new digestion will be performed tomorrow.
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Test digestion of pSB1C3_pHTERT</b></p>====
| |
| - | Investigator: <b>Bea </b>
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: pSB1C3_hTERT was sent for sequencing and revealed that the PCR and the inserting of the PCR product : promoter for hTERT was sucessfully performed. But the sequencing results showed a new AgeI restriciton site in the plasmid backbone. We could not see if the new recognition site was due to the bad sequence read or that there is really a new AgeI site. Therefore, a test digestion will be perfomed with PstI and AgeI in order to figure our wheter AgeI is present in the backbone or not.</p>
| |
| - | <br />
| |
| - | <p style="font-size:13px;"><b>Sequence analysis of pSB1C3_hTERT: </b></p>
| |
| - | <gallery widths=450px heights=300px perrow=2 caption="Sequencing results of pSB1C3_hTERT">
| |
| - | Image:Freiburg10 Sequence analysis of pSB1C3 hTERT suffix.jpg
| |
| - | Image:Freiburg10 Sequence analysis of pSB1C3 hTERT CAT mutations.jpg
| |
| - | </gallery>
| |
| - | <br />
| |
| - | <br />
| |
| - | <b>First the sequenced plasmid P177 was digested:</b>
| |
| - | <br />
| |
| - | <table border=1 cellpadding=1 cellspacing=1 width=284 style='border-collapse:
| |
| - | collapse;table-layout:fixed;width:214pt'>
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - | <td height=30 class=xl6312733 width=142 style='height:22.5pt;width:107pt'> </td>
| |
| - | <td class=xl6412733 width=142 style='border-left:none;width:107pt'>P177/µL</td>
| |
| - | </tr>
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - |
| |
| - | <td height=30 class=xl6512733 width=142 style='height:22.5pt;border-top:none;
| |
| - | width:107pt'>DNA</td>
| |
| - | <td class=xl6612733 style='border-top:none;border-left:none'>3</td>
| |
| - | </tr>
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - | <td height=30 class=xl6512733 width=142 style='height:22.5pt;border-top:none;
| |
| - | width:107pt'>BSA (10x)</td>
| |
| - | <td class=xl6612733 style='border-top:none;border-left:none'>1,5</td>
| |
| - | </tr>
| |
| - |
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - | <td height=30 class=xl6512733 width=142 style='height:22.5pt;border-top:none;
| |
| - | width:107pt'>Buffer 4<span style='mso-spacerun:yes'> </span>(10x)</td>
| |
| - | <td class=xl6612733 style='border-top:none;border-left:none'>1,5</td>
| |
| - | </tr>
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - | <td height=30 class=xl6512733 width=142 style='height:22.5pt;border-top:none;
| |
| - | width:107pt'>Enzyme PstI</td>
| |
| - |
| |
| - | <td class=xl6612733 style='border-top:none;border-left:none'>0,5</td>
| |
| - | </tr>
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - | <td height=30 class=xl6512733 width=142 style='height:22.5pt;border-top:none;
| |
| - | width:107pt'>Enzyme AgeI</td>
| |
| - | <td class=xl6612733 style='border-top:none;border-left:none'>0,5</td>
| |
| - | </tr>
| |
| - | <tr height=30 style='mso-height-source:userset;height:22.5pt'>
| |
| - |
| |
| - | <td height=30 class=xl6512733 width=142 style='height:22.5pt;border-top:none;
| |
| - | width:107pt'>H2O</td>
| |
| - | <td>8</td>
| |
| - | </tr>
| |
| - | <tr height=30;height:22.5pt'>
| |
| - | <td height=30 width=142 style='height:22.5pt;
| |
| - | width:107pt'><b>Total volume</b></td>
| |
| - | <td><b>15</b></td>
| |
| - | </tr>
| |
| - |
| |
| - | <tr height=0 style='display:none'>
| |
| - | <td width=142 style='width:107pt'></td>
| |
| - | <td width=142 style='width:107pt'></td>
| |
| - | </tr>
| |
| - | </table>
| |
| - | <br />
| |
| - | <ul>
| |
| - | <li>Incubation time: 45minutes</li>
| |
| - | <li>Incubation tempereature: 37°C</li>
| |
| - | </ul>
| |
| - | After the incubation,the samples were loaded on the 1% agarose gel, and run at 110 V for 45 minutes.
| |
| - | <br />
| |
| - | <br />
| |
| - | <b>Result of the test digestion:</b>
| |
| - | <br />
| |
| - | <gallery widths=400px heights=300px caption="Test digestion of pSB1C3_hTERT">
| |
| - | Image: Freiburg10 Testdigestion pSB1C3 hTERT 14 08.jpg
| |
| - | </gallery>
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#33cc33;"><b>Results:</b> As it can be seen in the sequencing results of pSB1C3_hTERT there could have been an additional AgeI site in the vector. If AgeI would have been in the vector, two fragments have been expected by digetsing the plasmid with AgeI and PstI. As it can be seen in the agarose gel, lane 11 (labeled with the green P177) only reveals one lane instead of two. Therefore it can be assumed that NO AgeI site is in the pSB1C3 backbone.</p>
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Picking clones ... </b></p>====
| |
| - | Investigator: Patrick
| |
| - | 15 Clones were picked saturday night so that a mini-prep could be performed on sunday.
| |
| - |
| |
| - | === 90. labday 15.08.2010===
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Sequence analysis of pSB1C3_Rep52</b></p>====
| |
| - | <b>Investigator: Bea </b>
| |
| - | <br />
| |
| - | <p style="font-size:13px; color:#003399;"><b>Comments</b>: Clone 3 was sent for sequencing because this clone revealed the right band sizes of the test digestion performed friday. For more details see: [http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/index.php/Laborjournal#Mini-Prep_and_Test_digestion_of_Rep40_and_Rep52_of_the_cloning_experiment_.28.22Cloning_of_Rep40.2F52.22_11.08.2010_Investigator:_Anna.29 Test digestion] </p>
| |
| - |
| |
| - | <gallery widths=700px heights=300px caption="Sequencing results of pSB1C3_Rep52 clone 3">
| |
| - | Image:Freiburg10 Sequence analysis of pSB1C3 Rep52 14 08 2010.JPG
| |
| - | </gallery>
| |
| - | <br />
| |
| - | <b>Results:</b> <p style="color:#00bbff;"> Sequence assembly showed that the suffix of the Rep protein 52 is ok and that PCR worked well. </p> <p style="color:#990000;">There can be seen that a silent mutation from AGG to AGA was inserted, but it will have no effect on the protein because codon usage in <i>H. sapiens </i>is nearly the same for both codons.</p>
| |
| - | The prefix could not be analysed because of bad sequence read quality. For further verification, another sequencing with the forward primer has to be performed.
| |
| - | <br />
| |
| - | <b>Next steps: Send plasmid for another sequencing round with forward primer in order to analyse the prefix. </b>
| |
| - | <br />
| |
| - | <br />
| |
| - | [http://www.molbiotech.uni-freiburg.de/iGEM/wiki2010/index.php/Laborjournal top of page]<br />
| |
| - |
| |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Mini-Prep of pSB1C3_Rep68, pSB1C3_Rep78, pSB1C3_RepAAP, pSB1C3_Rep40, pSB1C3_Rep52</b></p>====
| |
| - | <b>Investigator: Stefan</b><br>
| |
| - | <ul>
| |
| - | <b>Glycerol stocks were prepared:</b>
| |
| - | <li>B185 = pSB1C3_Rep68 clone 1
| |
| - | <li>B186 = pSB1C3_Rep68 clone 2
| |
| - | <li>B187 = pSB1C3_Rep68 clone 3<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>B188 = pSB1C3_Rep78 clone 1
| |
| - | <li>B189 = pSB1C3_Rep78 clone 2
| |
| - | <li>B190 = pSB1C3_Rep78 clone 3<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>B191 = pSB1C3_AAP clone 1
| |
| - | <li>B192 = pSB1C3_AAP clone 2
| |
| - | <li>B193 = pSB1C3_AAP clone 3<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>B194 = pSB1C3_Rep40 clone 1
| |
| - | <li>B195 = pSB1C3_Rep40 clone 2<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>B196 = pSB1C3_Rep52 clone 1
| |
| - | <li>B197 = pSB1C3_Rep52 clone 2<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>B198 = pSB1C3_SDM_SspI_Bla14FM clone 4
| |
| - | <li>B199 = pSB1C3_SDM_SspI_Bla14FM clone 5<br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - |
| |
| - |
| |
| - | <b>Mini-Prep following the standard protokol</b>
| |
| - | <br />
| |
| - | <li>P208 = pSB1C3_Rep68 clone 1 c=230,0
| |
| - | <li>P209 = pSB1C3_Rep68 clone 2 c=160,5
| |
| - | <li>P210 = pSB1C3_Rep68 clone 3 c=128,6 <br />
| |
| - | <br />
| |
| - |
| |
| - | <li>P211 = pSB1C3_Rep78 clone 1 c=150,8
| |
| - | <li>P212 = pSB1C3_Rep78 clone 2 c=108,6
| |
| - | <li>P213 = pSB1C3_Rep78 clone 3 c=131,3<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>P214 = pSB1C3_AAP clone 1 c=142,1
| |
| - | <li>P215 = pSB1C3_AAP clone 2 c=148,3
| |
| - | <li>P216 = pSB1C3_AAP clone 3 c=163,0<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>P217 = pSB1C3_Rep40 clone 1 c=164,5
| |
| - | <li>P218 = pSB1C3_Rep40 clone 2 c=<145,1br />
| |
| - | <br />
| |
| - |
| |
| - | <li>P219 = pSB1C3_Rep52 clone 1 c=156,0
| |
| - | <li>P220 = pSB1C3_Rep52 clone 2 c=164,0<br />
| |
| - | <br />
| |
| - |
| |
| - | <li>P221 = pSB1C3_SDM_SspI_Bla14FM clone 4 c=220,4
| |
| - | <li>P222 = pSB1C3_SDM_SspI_Bla14FM clone 5 c=252,2<br />
| |
| - | </ul>
| |
| - | <br />
| |
| - | <br />
| |
| - | <br />
| |
| - | <b>Test digestion:</b> <br />
| |
| - | No test digestion for pSB1C3_SDM_SspI_Bla14FM clone 4 and 5.<br />
| |
| - | For test digestion, pSB1C3_leftITR_CMV_mVenus (P206-207) and pSB1C3_leftITR_CMV (P188) was digested as well. <br />
| |
| - | Samples P208-220 were digested with XbaI and SpeI.<br />
| |
| - | Samples P207, P207 and P188 were digested with XbaI and NgoMIV.<br />
| |
| - |
| |
| - |
| |
| - | Gel used:<br />
| |
| - | 100 ml TAE (1x), 1 g agarose, 6 µl GELRED<br />
| |
| - | 115 V, 50 minutes
| |
| - | {| border="1"
| |
| - | | '''Ingredients''' || '''P208/µl'''|| |'''P209/µl''' || |'''P210/µl'''|| '''P211/µl'''|| |'''P212/µl''' || '''P213/µl'''|| |'''P214/µl''' || '''P215/µl'''|| |'''P216/µl''' || '''P217/µl'''|| |'''P218/µl''' || '''P219/µl'''|| |'''P220/µl''' || '''P206/µl'''|| |'''P207/µl''' || |'''P188/µl'''
| |
| - | |-
| |
| - | | DNA: || |4|| |6 || |7 || |6 || |9 || |7 || |6 || |6 || |6|| |6 || |6 || |6 || |6 || |3,5 || |3,5 || |4
| |
| - | |-
| |
| - | | BSA (10x): || |2 || |2 || |2|| |2 || |2 || |2|| |2 || |2 || |2|| |2 || |2 || |2|| |2 || |2 || |2|| |2
| |
| - | |-
| |
| - | | Buffer 4: || |2 || |2 || |2|| |2 || |2 || |2|| |2 || |2 || |2|| |2 || |2 || |2|| |2 || |2 || |2|| |2
| |
| - | |-
| |
| - | | Enzyme 1 || |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5
| |
| - | |-
| |
| - | | Enzyme 2 || |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5 || |0,5 || |0,5|| |0,5
| |
| - | |-
| |
| - | |H<sub>2</sub>O|| | 11|| | 9|| |8|| | 9|| | 6|| |8|| | 9|| | 9|| |9|| | 9|| | 9|| |9|| | 9|| | 11,5|| |11,5|| | 11
| |
| - | |-
| |
| - |
| |
| - | |}
| |
| | | | |
| - | <br />
| + | ===17. Labortag 31.05.2010=== |
| | | | |
| - | <br /> | + | ====<p style="font-size:15px; background-color:#66bbFF;"><b>pAAV_RC R585A and R588A transistions</b></p>==== |
| - | [[File:Freiburg10 Rep40-78 mVenus.jpg|700px]]
| + | |
| - | <br /> | + | |
| - | <br /> | + | |
| - | <br /> | + | |
| - | comment: Rep- and AAP approaches have to be repeated. We did not realize that the vector can religate! Plasmids and glycerol stocks were thrown away.
| + | |
| | | | |
| - | ====<p style="font-size:15px; background-color:#66bbff;"><b>Inoculation of CD colony</b></p>====
| |
| - | Investigator: Kira
| |
| | | | |
| - | One colony was picked from agar plate and dispersed into 10 ml DYT+ Amp and incubated over night @ 37 C.
| |
| | | | |
| | + | In order to alter the tropism of AAV2 several modifications have to be performed. |
| | + | First of all the binding to Heparan Sulfate Proteoglycan has to be disrupted. This can be done by R585A and R588A transistions: |
| | + | [[File:Freiburg10 R585A R588A.jpg|800px|]] |
| | | | |
| | <html> | | <html> |
| | </div> | | </div> |
| | </html> | | </html> |

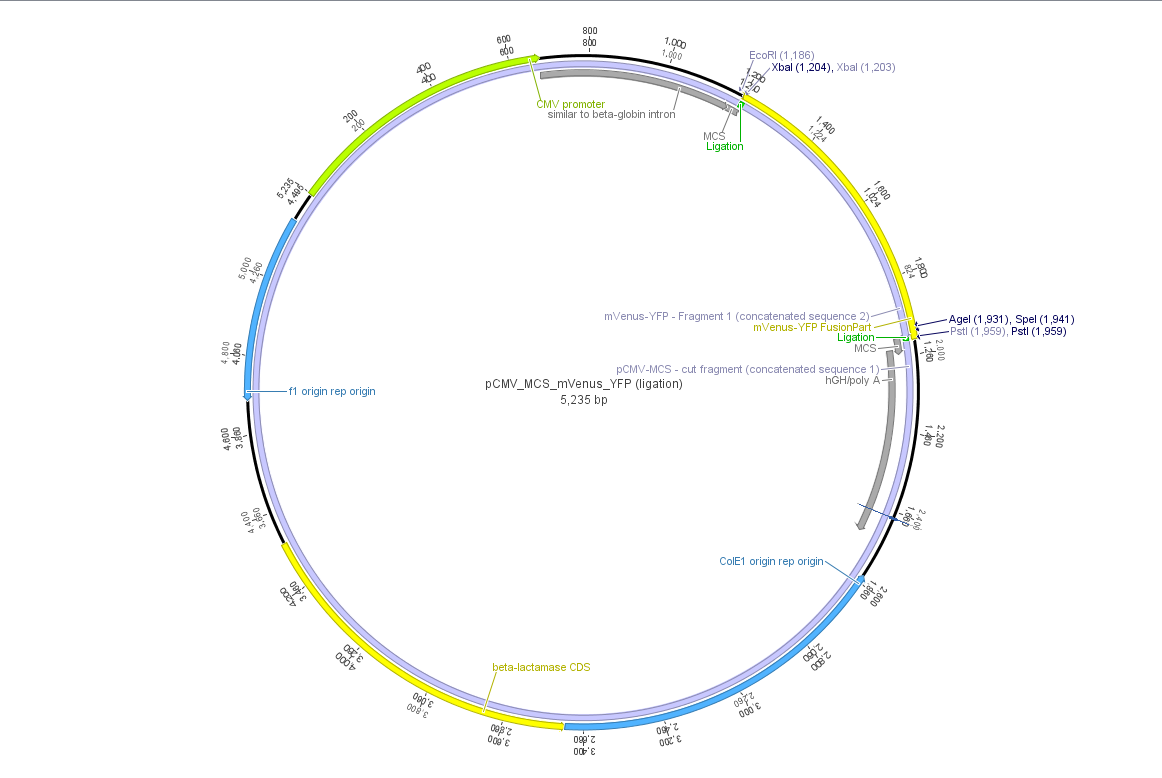

 "
"