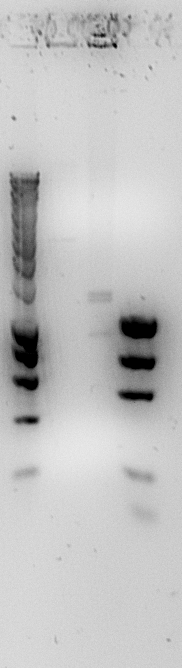Team:TU Delft/19 August 2010 content
From 2010.igem.org
(→Alkane Degradation) |
(→Colony PCR) |
||
| Line 119: | Line 119: | ||
|- | |- | ||
|2 | |2 | ||
| - | |transformant #1 of ligation mix: | + | |transformant #1 of ligation mix: ‘E - J61101-ADH-pSB1A2 - X'+ |
|1239 | |1239 | ||
|G00101 + G00101 | |G00101 + G00101 | ||
Revision as of 15:25, 31 August 2010
Contents |
Software development
By Jelmer
Part of the dry lab work involves developing an application that can suggest possible interactions between the newly introduced proteins, and the existing E. coli proteome. This page will be updated with the application, documentation and results of applying it to our biobricks.
The basic idea is to look for interactions of the proteins within their own organism, and map those interactions by looking at homologs in E. coli:
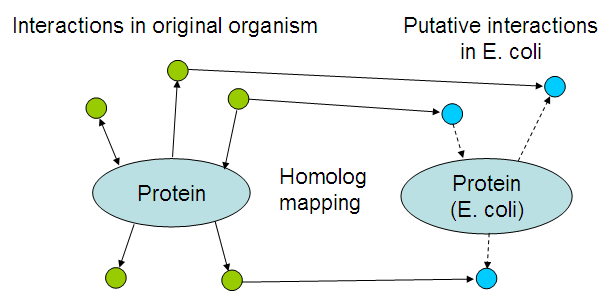
The resulting list of interactions might be usable to 'debug' the resulting bacteria, i.a.w. suggest reasons why the system is not working like it should. Even though there would be no time to fix those problems, it could still help teams in following years that want to take another stab at solving the problem.
Today the work on this application resulted in the first list of some potentially interacting proteins. A locally running version of the [http://string-db.org STRING protein database] (~150 gb PostgreSQL database) was used to look for interactions and homologs . Ideally however, this application would work using existing biological databases. I'm still looking for the right webservices to accomplish this, they might not exist.
Alkane Degradation
Digestion
The results from sequencing are in! It appears that K398013 is not exactly as we expected it to be. A new method of making 013 (however without the terminator region) will be attempted by ligating 009 and 010 digestion products:
| # | Sample | Enzyme 1 | Enzyme 2 | Enzyme 3 | Buffer | BSA | Needed fragment |
| 1 | 009A | SpeI | PstI | None | NEBuffer 2 | ✓ | ‘S - J23100-J61100-RubA4-pSB1A2 - P’ |
| 2 | 010A | XbaI | PstI | AseI | NEBuffer 3 | ✓ | ‘X - J61100-RubR - P’ |
The digestion mixes were incubated for 1 hr. at 37 degrees and partially loaded onto a 1% agarose gel:
Lane description
| # | Description | Expected size (bp) | OK? |
| 1 | Smartladder | Varies | Yes |
| 2 | 009A cut | 2309, 14 | Yes (vague) |
| 3 | 010A cut | 1246, 1185, 872, 43 | Yes |
| 4 | EZ Load | Varies | Yes |
Ligation
Following the digestion the products were ligated for 1 hr. at 20 degrees:
| # | BioBrick | Fragment 1 | Fragment 2 |
| 1 | 014C | ‘S - J23100-J61100-RubA4-pSB1A2 - P’ | ‘X - J61100-RubR - P’ |
Transformation
The ligation mix was trasnformed into chemically competent TOP10 cells and plate out onto AMP plates.
Colony PCR
Yesterday's transformants were checked for positives by cPCR. Furthermore, possible K398020 (see blog of August 12th) and K398406 transformants were checked simultaneously.
Lane description:
| # | Description | Expected Length [bp] | Primers | Status | Remarks |
| 1 | SmartLadder | Varies | None | Good | None |
| 2 | transformant #1 of ligation mix: ‘E - J61101-ADH-pSB1A2 - X'+ | 1239 | G00101 + G00101 | ? | |
| 3 | transformant #2 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 4 | transformant #3 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 5 | transformant #4 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 6 | transformant #5 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 7 | transformant #1 of ligation mix: E-pSB1AK2-I13401-X (ligation control) | 1173 | G00101 + G00101 | ? | |
| 8 | transformant #2 of ligation mix: E-pSB1AK2-I13401-X (ligation control) | 1173 | G00101 + G00101 | ? | |
| 9 | transformant #3 of ligation mix: E-pSB1AK2-I13401-X (ligation control) | 1173 | G00101 + G00101 | ? | |
| 10 | transformant #1 of digestion mix: E-pSB1AK2-I13401-X (digestion control) | 1173 | G00101 + G00101 | ? | |
| 11 | transformant #2 of digestion mix: E-pSB1AK2-I13401-X (digestion control) | 1173 | G00101 + G00101 | ? | |
| 12 | transformant #1 of K081005 in pSB1A2 | 296 | G00101 + G00101 | ? | |
| 13 | transformant #2 of K081005 in pSB1A2 | 296 | G00101 + G00101 | ? | |
| 14 | transformant #3 of K081005 in pSB1A2 | 296 | G00101 + G00101 | ? | |
| 15 | BioRad EZ Load | n/a | n/a | n/a | |
| 16 | Digested PCR product of E0240, XbaI and PstI | 898 | None | ? | |
| 17 | Digested PCR product of E0240, XbaI and PstI | 898 | None | ? | |
| 18 | Digested PCR product of E0240, XbaI and PstI | 898 | None | ? |
Lane description:
| # | Description | Expected Length [bp] | Primers | Status | Remarks |
| 1 | SmartLadder | Varies | None | Good | None |
| 2 | transformant #1 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 3 | transformant #2 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 4 | transformant #3 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 5 | transformant #4 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 6 | transformant #5 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ? | |
| 7 | transformant #1 of ligation mix: E-pSB1AK2-I13401-X (ligation control) | 1173 | G00101 + G00101 | ? | |
| 8 | transformant #2 of ligation mix: E-pSB1AK2-I13401-X (ligation control) | 1173 | G00101 + G00101 | ? | |
| 9 | transformant #3 of ligation mix: E-pSB1AK2-I13401-X (ligation control) | 1173 | G00101 + G00101 | ? | |
| 10 | transformant #1 of digestion mix: E-pSB1AK2-I13401-X (digestion control) | 1173 | G00101 + G00101 | ? | |
| 11 | transformant #2 of digestion mix: E-pSB1AK2-I13401-X (digestion control) | 1173 | G00101 + G00101 | ? | |
| 12 | transformant #1 of K081005 in pSB1A2 | 296 | G00101 + G00101 | ? | |
| 13 | transformant #2 of K081005 in pSB1A2 | 296 | G00101 + G00101 | ? | |
| 14 | transformant #3 of K081005 in pSB1A2 | 296 | G00101 + G00101 | ? | |
| 15 | BioRad EZ Load | n/a | n/a | n/a | |
| 16 | Digested PCR product of E0240, XbaI and PstI | 898 | None | ? | |
| 17 | Digested PCR product of E0240, XbaI and PstI | 898 | None | ? | |
| 18 | Digested PCR product of E0240, XbaI and PstI | 898 | None | ? |
Ligation Kinetics
The results of the ligation efficiencies at varying times were as follows:
| # | Incubation time [min] | # colonies |
| 1 | 30 | 64 |
| 2 | 60 | 100 |
| 3 | 120 | 80 |
| 4 | 240 | 60 |
The digestion control plate contained 1 colony, indicating a negligible background. From this experiment we can thus conclude that a 1 hr. ligation is long enough to obtain sufficient transformants.
 "
"