|
AUGUST: WEEK 4
August, 23rd
Colony PCR as screening for ligations I31..I41. For each plate three colonies were picked and PCR amplification was performed. At the same time colonies were let grow in 750 ml LB+Amp in order to be ready to make glycerol stocks for those with right amplicons.
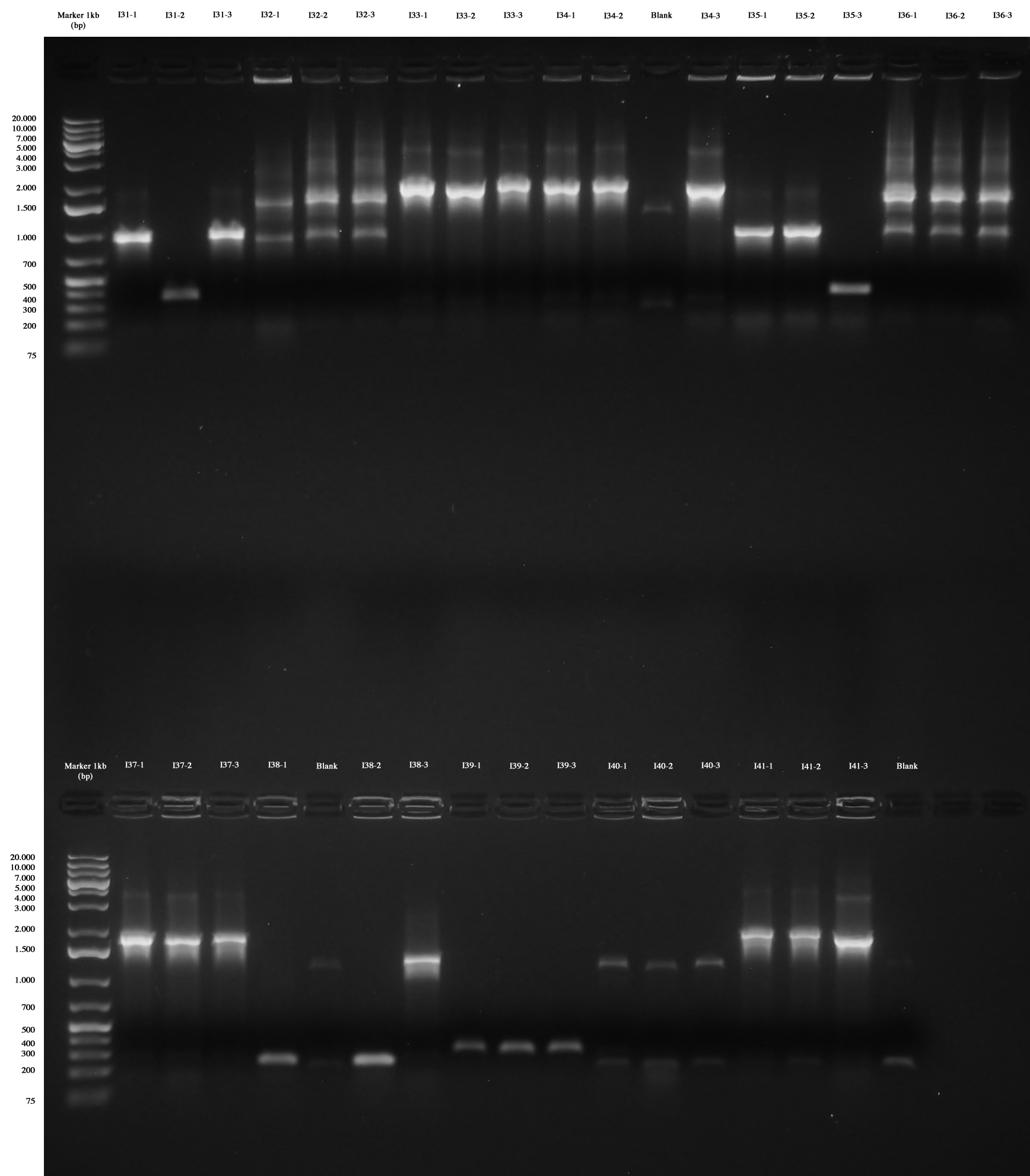 Colony PCR and gel run as screening for ligations I31..I41 Only I31, I33, I34, I35, I37, I38, I41 are positive without any doubt (we took I31-1, I33-1. I34-1, I35-1, I37-1, I38-3, I41-1), while I39 were all wrong and we weren't sure about I32, I36 and I40 (we made however glycerol stocks for I32-2, I36-2, I40-1).
We decided to repeat colony PCR for I32, I36, I39 and I40 next day.
August, 24th
Only this morning we remembered that I40 is in a commercial vector (pMA), we will screen it next day through an E-P digest (today we made glycerol stocks for other four colonies: I40-4/5/6/7) since it isn't possible with colony PCR.
With this method we checked four new colonies of I32 (I32-4/5/6/7), I36 (I36-4/5/6/7) and I39 (I39/4/5/6/7).
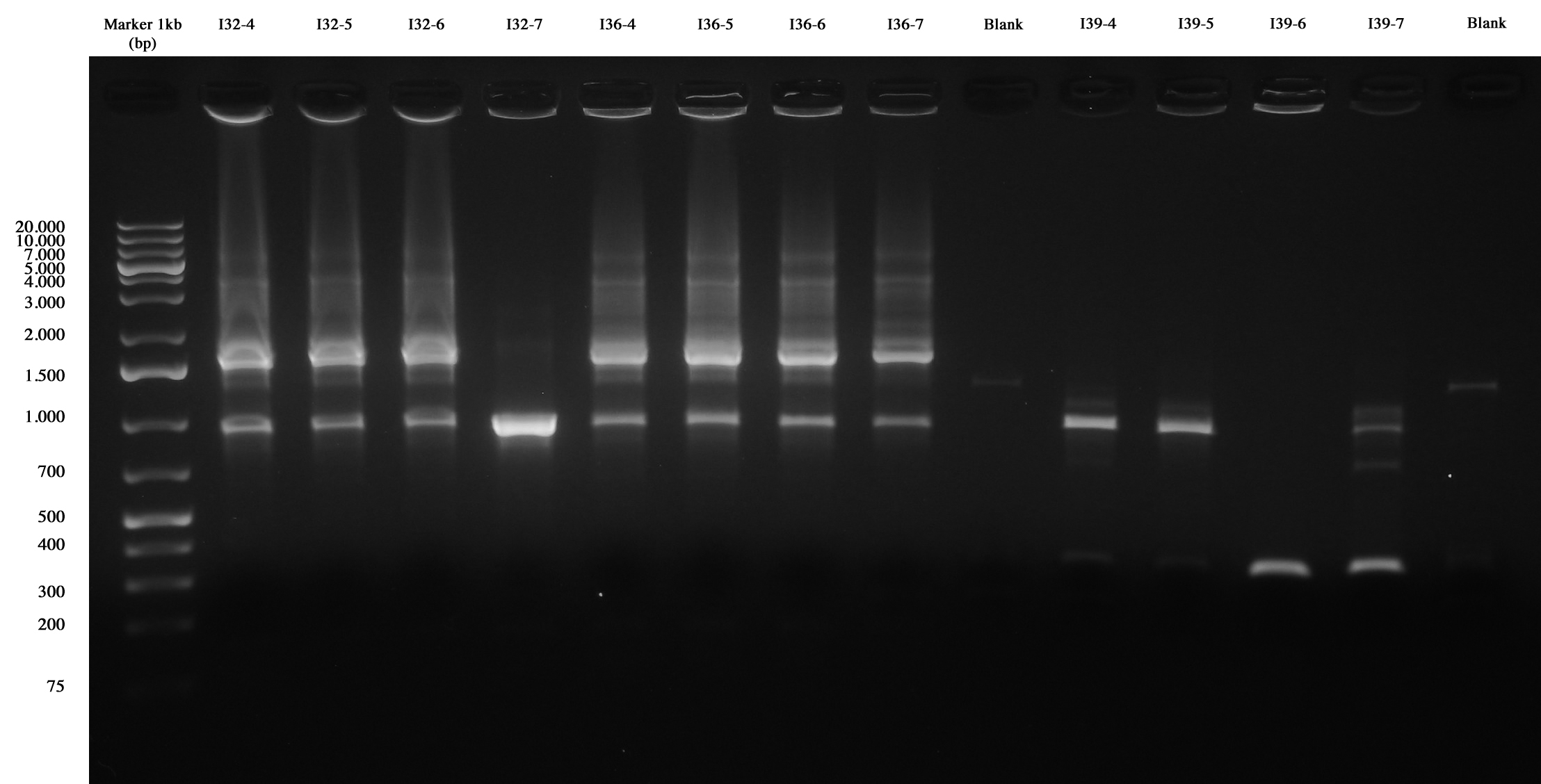 Colony PCR and gel run as screening for ligations I32, I36, I39 I39-4/5 are right (we made glycerol stock for I39-5); I32 and I36 still show the presence of cotrasformation of our ligation and a simple phasin (we took I36-7 instead of I36-2: cleaner gel run). We inoculated 5ml LB+Amp with I40-1/4/5/6/7 and we let them grow ON at 37°C, 220 rpm for E-P digestion/screening.
Inoculum of:
- <partinfo>BBa_J23105</partinfo>
- <partinfo>BBa_J23106</partinfo>
- <partinfo>BBa_J23114</partinfo>
- <partinfo>BBa_J23116</partinfo>
for tomorrow MiniPrep. They will be processed with other promoters, for wich we retrieved purified DNA from our freezer. These further parts are:
- <partinfo>BBa_J23100</partinfo>
- <partinfo>BBa_J23101</partinfo> already digested E-P and purified
- <partinfo>BBa_J23110</partinfo>
- <partinfo>BBa_J23118</partinfo>
- <partinfo>pSB4C5</partinfo> already digested E-P and purified
These promoters, expressing RFP, will be moved from high copy plasmid <partinfo>pSB1A2</partinfo> to the low copy plasmid <partinfo>pSB4C5</partinfo> and will be tested in both condition in order to establish a strength ranking.
August, 25th
Minipreps of I40-1/4/5/6/7 (to make the screening of ligations) were quantified as follows:
- I40-1: 415,7 ng/ul
- I40-4: 382,8 ng/ul
- I40-5: 420 ng/ul
- I40-6: 487,8 ng/ul
- I40-7: 479,9 ng/ul
Samples were digested E-P for three hours
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 (ul) | Enzyme 2 (ul) | Buffer H (ul)
|
| I40-1 | Insert/Screening | 25 | 2 | 19,5 | 0,5 EcoRI | 0,5 PstI | 2,5
|
| I40-4 | Insert/Screening | 25 | 2 | 19,5 | 0,5 EcoRI | 0,5 PstI | 2,5
|
| I40-5 | Insert/Screening | 25 | 2 | 19,5 | 0,5 EcoRI | 0,5 PstI | 2,5
|
| I40-6 | Insert/Screening | 25 | 2 | 19,5 | 0,5 EcoRI | 0,5 PstI | 2,5
|
| I40-7 | Insert/Screening | 25 | 2 | 19,5 | 0,5 EcoRI | 0,5 PstI | 2,5
|
and than gel run to check the length of ligations.
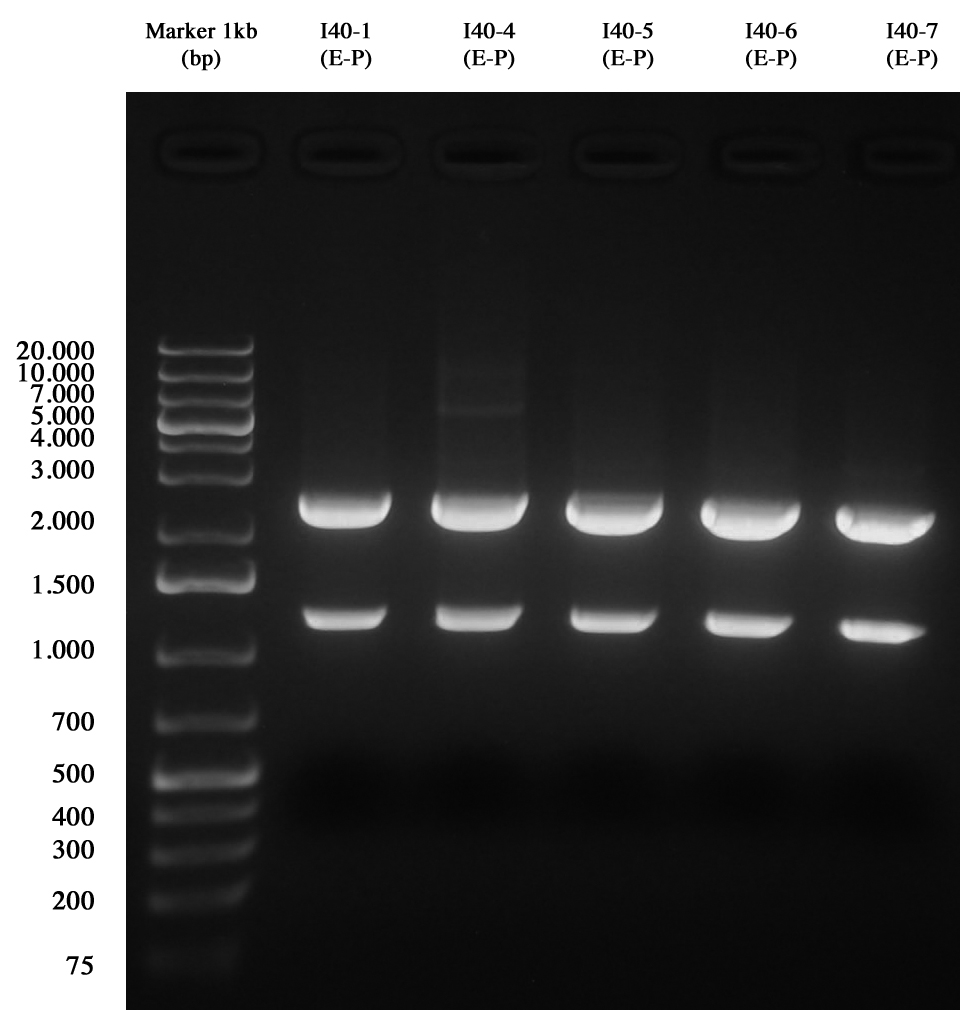 Gel run for I40-1/4/5/6/7 digested E-P As you can see all samples are positive, so we decided to keep glycerol stock of I40-1.
MiniPrep was performed for following cultures, and DNA was quantified as follows:
| Culture name | Quantifiaction (ng/ul)
|
| <partinfo>BBa_J23105</partinfo> | X ng/ul
|
| <partinfo>BBa_J23106</partinfo> | X ng/ul
|
| <partinfo>BBa_J23114</partinfo> | X ng/ul
|
| <partinfo>BBa_J23116</partinfo> | X ng/ul
|
Purified DNA was digested as follows:
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| <partinfo>BBa_J23100</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_J23105</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_J23106</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_J23110</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_J23114</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_J23116</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
| <partinfo>BBa_J23118</partinfo> | Insert | 25 | 10 | 10,5 | 1 EcoRI | 1 PstI | 2,5
|
Ligation of:
- <partinfo>BBa_J23100</partinfo>_4C5=<partinfo>BBa_J23100</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23101</partinfo>_4C5=<partinfo>BBa_J23101</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23105</partinfo>_4C5=<partinfo>BBa_J23105</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23106</partinfo>_4C5=<partinfo>BBa_J23116</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23110</partinfo>_4C5=<partinfo>BBa_J23110</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23114</partinfo>_4C5=<partinfo>BBa_J23114</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23116</partinfo>_4C5=<partinfo>BBa_J23116</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
- <partinfo>BBa_J23118</partinfo>_4C5=<partinfo>BBa_J23118</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>(E-P)
Inoculum of self inducible promoters for tomorrow TECAN test.
Preparation of samples for BioPlastic screening (... dire bene di come sono stati preparati i vetrini, dopo 8h, nel controllo, nel non indotto, non indotto+gly, IPTG e IPTG+gly)
August, 26th
August, 27th
August, 28st
August, 29nd
|
|
 "
"



