|
Week 5: Monday 9th - Sunday 15th August
Monday
We took photographs of the petri dishes that Theo and Peter had drawn. These had grown up well, over a short period. We believe this is because they were plated out with only ampicillin, and no chloramphenicol.
Will designed oligos to be sent to Biolegio for synthesis. Hannah and Emily wrote more messages on dishes, and planned a restriction digest to check for the biobrick compatibility of the V. fischeri lux operon.
Anja and Theo miniprepped an overnight culture which had been inoculated with a ligation of an expression backbone and firefly luciferase. They ran it on a gel, which showed that the insert had worked. Discovered that E-gels can be used multiple times. Then they performed a very bodged luciferase assay. 0.5ml of cells were frozen and thawed three times to lyse them, then 0.05ml of a concentrated D-luciferin solution was added. The camera showed them to be glowing.
Tuesday
Will constructed oligos, and taught Emily and Hannah to do so.
V. phosphoreum arrived, Anja and Theo made up its specialised, salty culture. Peter and Theo placed it in five subcultures and inoculated.
Wednesday
Our brand new, fancy plate reader arrived on today from BMG for use to use for the next 2 months. We had a tutorial and are really looking forward to actually using it.
Emily and Hannah spent all day designing Phosphoreum oligos. It messed with our heads. Hannah became quite hysterical. Will designed oligos for site-directed mutagenesis of Phosphoreum. Emily sent off the V. fischeri oligo order.
In the evening we had a team social murder mystery. Peter won the best actor prize, and Bill did it!
Thursday
In the morning we had an e-mail saying we had slightly underestimated how much our oligo order would cost - Mike from Biolegio asked if we really wanted to spend £1200. Oops! So, we started re-thinking the order and deciding exactly which oligos we needed for the experiments we wanted to do. We also decided what we wanted to ask people about in the lab meeting in the afternoon.
In the afternoon, we had a lab meeting. We need to think more about what our 'big idea' is and plan out some more experiments.
Friday
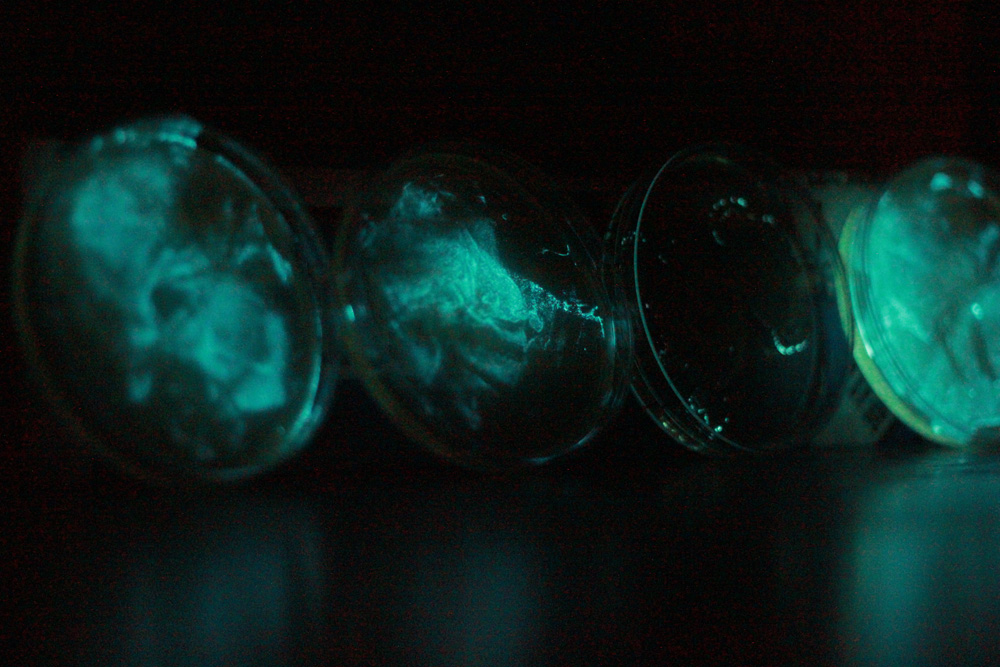
Phosphoreum are glowing!
Will and Emily finished the re-designed V. fischeri oligos and sent them off to biolegio to be synthesised. Hannah started designing experiments to do with the oligos. Peter has been looking into oligos for quiescence. Bill has been transforming cells with the Pbad promoter from the registry. Will also grew up some cells so that we can extract plasmids to use oligos with.
Theo wrote a script to make the BMG plate reader take concurrent OD and luminescence reading over time, we set it going overnight. He also wrote a script in Python to process its output and spread some V. phosphoreum on plates. Theo also streaked out some lumazine (BBa_K216007) and luxAB (BBa_K216008)
Saturday
Theo and Peter came in, Theo confirmed that the script had measured OD successfully and set it going again with a longer timeframe. Luminescence results were unclear.
The V. phosphoreum had formed a thin film which was glowing brightly. Peter streaked it out in an attempt to get single colonies and did much needed tidying.
Theo examined the lumazine and luxAB colonies. Lumazine was unexpectedly pink, both were streaked out again to get better colonies. The plate reader was set running again for the rest of the weekend.
Sunday
We took our first photo of a person lit entirely by bioluminescence. Could this be a taste of a possible future?
Theo also tried to get P. phosphoreum glowing on Sainsbury's prawns, but they did not seem to appreciate them, despite their quality. (Taste the Difference no less)
|
Monday
31. Experiment: Diagnostic gel to test for luciferase insertion (Theo & Anja)
Took overnight culture of TOP10 transformed with plasmid (potentially) containing promoter + rbs and luciferase
Plasmid DNA purification
following "QIAprep Spin Miniprep Kit using a Microcentrifuge" protocol
Gel electrophoresis
E-gel EX Agarose 1% mounted on transilluminator
Nanodrop readings:
- Plasmid with promoter + rbs + luciferase: 18.5ng/μl => 5μl gives 100ng
- Plasmid with promoter + rbs only: 64.9ng/μl => 2μl gives 100ng
Gel loading mixture:
|
| promoter + rbs + luciferase
| promoter + rbs only
|
| 6X orange loading Dye
| 3μl
| 3μl
|
| plasmid DNA
| 5μl
| 2μl
|
| deionised H2O
| 12μl
| 12μl
|
Supercoiled DNA ladder loading mixture
- 1μl Supercoiled DNA ladder (NEB)
- 3μl 6X orange loading Dye
- 16μl deionised H2O
Gel was loaded following scheme below:
| Supercoiled DNA ladder
| promoter + rbs only
| promoter + rbs + luciferase
|
| 20μl
| 20μl
| 20μl
|
Empty wells were filled with 200μl deionised H2O. Gell was run and a clear band around 2kb was visible for promoter + rbs, a very faint band was observed around 4kb for promoter + rbs + luciferase.
Gel electrophoresis
was repeated with increased promoter + rbs + luciferase plasmid concentration
Gel loading mixture for promoter + rbs + luciferase
(others as above)
- 3μl 6X orange LD
- 15μl plasmid DNA
- 2μl deionised H2O
Gel was run and the same bands were observed as above, with the ~4kb promoter + rbs + luciferase more clearly than before
32. Experiment: In vitro assay for luciferase activity (Theo & Anja)
50μl of TOP10 cells transformed with plasmid containing promoter + rbs + luc were transferred to an Eppendorf tube and subjected to three rounds of freeze (-80°C) and thaw (37°C) treatement.
50μl of 2mg/ml luciferin were added tot he lysed bacteria and luminescence was tested using a CCD camera.
The experiment was performed twice in a row and both times luminescence was observed with the CCD camera (though not by eye in the dark room).
Tuesday
33. Experiment: In vitro assay for luciferase activity using CHBT as substrate (Theo and Anja)
2ml of TOP10 with promoter, rbs and luc were spun at 13000rpm for 5 mins. Supernatant was discarded and cell pellet was resuspended in 500μl fresh LB to which 1 drop of chloroform was added. The cells were subjected to three rounds of freeze (-80°C) and thaw (37°C) treatment.
EXPERIMENT WAS CANCELLED!
34. Experiment: Preparation of Photobacterium Broth (Theo and Anja)
35.2g broth promoter was dissolved in 500ml water by warming/boiling. Broth was filter sterilised.
35. Experiment: Ampoule of Photobacterium (extracted by Theo and Peter)
Ampoule was broken, 500μl LB broth added and 100μl added to each of 5 falcons containing broth.
Wednesday
Theo added 5g agar to 250ml broth and placed for autoclaving.
After autoclaving the medium was turbid. It was poured into 9 empty, sterile dishes and left to set on the bench.
Thursday
36. Experiment: Renewed colonies of TOP10/pHK555 & pHK724 (Will)
- Inoculated 2x5ml LB with 1 colony for each
- Streaked out cells on Amp + Chl plate (*)
- Incubated both at 30°C overnight.
Results:
- 1 LB glow very faintly, other not at all
- Poor growth from streaking, but strong glow
Friday
36. Experiment: Transformation of TOP10 competent cells with luxR from registry (Bill)
- Followed transformation protocol from 02/08/10
- Transformed with BBa_I0462 from distribution from distribution Kit Plate 1 Well 80
- Plated out on 2 ampicillin plates the transformation, (amp is resistance marker of plasmid)
- Also cultured in 2 LB + Amp plates
Results (Hannah):
| Plate/Broth
| Contains
| Growth
|
| Plate (Amp)
| TOP10 LuxR
| Yes
|
| Plate (Amp)
| TOP10 Only
| No
|
| Plate (No Amp)
| TOP10 LuxR
| Yes
|
| Broth (LB + Amp)
| TOP10 LuxR
| No
|
37. Experiment: Plating out of registry stabs (Theo)
Theo plated out BBa_K216007 and 08 on A plates
- 5ml SOB + 2.5μl Chloramphenicol + 1 colony Invitrogen 10 with pHK555 (from plate 5/8/10)
- 5ml SOB + 2.5μl Chloramphenicol + 10μl of glycerol stock of Slock10/pHK555 cells
- 5ml LB + 2.5μl Chloramphenicol + 1 colony Invitrogen 10 with pHK555 (from plate 5/8/10)
39. Experiment: Restreaked TOP10/pHK555+pHK724 (Will)
Using poorly grown strain from yesterday (*) restreaked onto LB agar+Chl+Amp (hope to select for strong growth).
40. Experiment: Streaked out Invitrogen 10 pHK555 (from plate 5/8/10) (Will)
On Chl plate, for colony PCR of pHK555 Plasmid
Saturday
41. Experiment: Culturing in broth of TOP10+LuxR from registry (Hannah)
- Took single colony from plate, added to LB+Amp broth
- 2 days later took them out and put in fridge
Sunday
|

 "
"