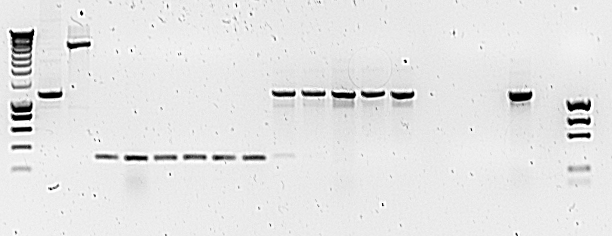Team:TU Delft/21 July 2010 content
From 2010.igem.org
(→Method 4) |
|||
| (13 intermediate revisions not shown) | |||
| Line 82: | Line 82: | ||
====Assembly of reference construct==== | ====Assembly of reference construct==== | ||
| + | |||
| + | =====Method 1===== | ||
| + | |||
| + | <i>Hypothesis II</i> | ||
| + | The transformants that were replated on kanamycin plates produced colonies, indicating that hypothesis II was invalid. To test whether these colonies did contain the proper insert however, a [https://2010.igem.org/Team:TU_Delft#page=protocols/colony_PCR colony PCR] was performed: | ||
| + | |||
| + | [[Image:TU_Delft_lastgel_21Jul10.png|450px|thumb|left|1% agarose of colony PCR. Gel runned at 100V for 1 hour. Of all samples 5 µL was loaded with 1 µL loadingbuffer. 5 µL was loaded of marker]] | ||
| + | |||
| + | Lane description: | ||
| + | {|style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| + | |'''#''' | ||
| + | |'''Description''' | ||
| + | |'''Expected Length (bp)''' | ||
| + | |'''Primers''' | ||
| + | |'''Status''' | ||
| + | |'''Remarks''' | ||
| + | |- | ||
| + | |1 | ||
| + | |SmartLadder | ||
| + | |n/a | ||
| + | |n/a | ||
| + | |<font color=limegreen>✓</font> | ||
| + | | | ||
| + | |- | ||
| + | |2 | ||
| + | |PCR product of I13401 | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=limegreen>✓</font> | ||
| + | | | ||
| + | |- | ||
| + | |3 | ||
| + | |XbaI and PstI digested I13401 | ||
| + | |1239 | ||
| + | |None | ||
| + | |<font color=limegreen>✓</font> | ||
| + | |No additional bands visible (see hypothesis I) | ||
| + | |- | ||
| + | |4 | ||
| + | |Transformant #1 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |5 | ||
| + | |Transformant #4 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |6 | ||
| + | |Transformant #2 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |7 | ||
| + | |Transformant #3 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |8 | ||
| + | |Transformant #4 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |9 | ||
| + | |Transformant #5 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |10 | ||
| + | |transformant #6 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |11 | ||
| + | |Transformant #1 of ligation mix: E-pSB1AK3-I13401-X (ligation control) | ||
| + | |1173 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |12 | ||
| + | |Transformant #2 of ligation mix: E-pSB1AK3-I13401-X (ligation control) | ||
| + | |1173 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |13 | ||
| + | |Transformant #3 of ligation mix: E-pSB1AK3-I13401-X (ligation control) | ||
| + | |1173 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |14 | ||
| + | |transformant #1 of digestion mix: E-pSB1AK3-I13401-X (digestion control) | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |15 | ||
| + | |transformant #11 of ligation mix: E-pSB1AK3-I13401-X (digestion control) | ||
| + | |1239 | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |16 | ||
| + | |I13600 in pSB1A2 transformant #1 | ||
| + | | | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |17 | ||
| + | |I13600 in pSB1A2 transformant #2 | ||
| + | | | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |18 | ||
| + | |I13600 in pSB1A2 transformant #3 | ||
| + | | | ||
| + | |G00101 + G00101 | ||
| + | |<font color=red>✗</font> | ||
| + | | | ||
| + | |- | ||
| + | |19 | ||
| + | |BioRad EZ Load | ||
| + | |N/A | ||
| + | |N/A | ||
| + | ||<font color=limegreen>✓</font> | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | =====Method 3===== | ||
| + | TOP10 competent cells were [https://2010.igem.org/Team:TU_Delft#page=protocols/transformation transformed] with yesterday's ligation product. | ||
| + | |||
| + | =====Method 4===== | ||
| + | |||
| + | The obtained colonies on ampicillin plates were screened for GFP fluorescence. No discernable fluorescence was seen under UV excitation. It was decided to perform a [https://2010.igem.org/Team:TU_Delft#page=protocols/colony_PCR single colony PCR] on 17 randomly selected colonies to check for the proper insert: | ||
| + | |||
| + | [[Image:TU_Delft_scPCR_21-07.png|500px|thumb|left|1% agarose of colony PCR. Gel run at 100V for 1 hour. Of all samples 5 µL was loaded with 1 µL loadingbuffer. 5 µL was loaded of marker]] | ||
| + | |||
| + | Lane description: | ||
| + | {|style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| + | |'''#''' | ||
| + | |'''Description''' | ||
| + | |'''Expected Length (bp)''' | ||
| + | |'''Primers''' | ||
| + | |'''Status''' | ||
| + | |'''Remarks''' | ||
| + | |- | ||
| + | |1 | ||
| + | |SmartLadder | ||
| + | |Varies | ||
| + | |n/a | ||
| + | |<font color=limegreen>✓</font> | ||
| + | | | ||
| + | |- | ||
| + | |2 | ||
| + | |PCR product of E0240 | ||
| + | |1114 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |3 | ||
| + | |transformant #1 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |4 | ||
| + | |transformant #2 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |5 | ||
| + | |transformant #3 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |6 | ||
| + | |transformant #4 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |7 | ||
| + | |transformant #5 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |8 | ||
| + | |transformant #6 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |9 | ||
| + | |transformant #7 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |10 | ||
| + | |transformant #8 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |11 | ||
| + | |transformant #9 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |12 | ||
| + | |transformant #10 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |13 | ||
| + | |transformant #11 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |14 | ||
| + | |transformant #12 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |15 | ||
| + | |transformant #13 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |16 | ||
| + | |transformant #14 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |17 | ||
| + | |transformant #15 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |18 | ||
| + | |transformant #16 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |19 | ||
| + | |transformant #17 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | ||
| + | |1157 | ||
| + | |G00100 + G00101 | ||
| + | |<font color=red>?</font> | ||
| + | | | ||
| + | |- | ||
| + | |20 | ||
| + | |BioRad EZ Load | ||
| + | |Varies | ||
| + | |N/A | ||
| + | |<font color=limegreen>✓</font> | ||
| + | |} | ||
| + | |||
| + | The 43 bp difference was not discernable from gel, and thus this gel is quite inconclusive concerning the presence of positive colonies. | ||
| + | |||
| + | ==Solvent Tolerance and Hydrocarbon Sensing== | ||
| + | |||
| + | ===Characteristic digestion of isolated plasmids=== | ||
| + | |||
| + | To determine whether the plasmids are correct. Characteristic cuts were made using the restriction enzyme PvuI. | ||
| + | |||
| + | {| style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| + | |'''#''' | ||
| + | |'''Sample''' | ||
| + | |''' Enzyme 1''' | ||
| + | |'''Buffer''' | ||
| + | |'''Needed fragments''' | ||
| + | |- | ||
| + | |1 | ||
| + | |1450 μg K398328T | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |252, 1647, 69, 36, 3246 | ||
| + | |- | ||
| + | |2 | ||
| + | |378 μg K398407T | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |2552 | ||
| + | |- | ||
| + | |3 | ||
| + | |716.5 μg Control (pSB1T3, B0015: this does not ligate) | ||
| + | |PvuII (this enzyme does not work here) | ||
| + | |Buffer II | ||
| + | |172, 2463 | ||
| + | |- | ||
| + | |4 | ||
| + | |245.4 μg K398300C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |654, 252, 1647, 69, 36, 2004 | ||
| + | |- | ||
| + | |5 | ||
| + | |1481.4 μg K398000C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |495, 2901 | ||
| + | |- | ||
| + | |6 | ||
| + | |1198.5 μg K398002C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |2243 | ||
| + | |- | ||
| + | |7 | ||
| + | |42.3 μg K398002C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |3120 | ||
| + | |- | ||
| + | |8 | ||
| + | |1612.8 μg K398200C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |558, 135, 177, 1887 | ||
| + | |- | ||
| + | |9 | ||
| + | |1633.3 μg K398201C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | |2529 | ||
| + | |- | ||
| + | |10 | ||
| + | |938.6 μg K398400C | ||
| + | |PvuII | ||
| + | |Buffer II | ||
| + | | | ||
| + | |} | ||
| + | |||
| + | |||
| + | Results of the digestion on [[Team:TU_Delft/protocols/agarose_gel|1% agarose gel]] | ||
| + | |||
| + | [[Image:TU DELFT knipjes plasmiden.JPG|500px|thumb|left|1% agarose of plasmid check using digestion reaction. Gel runned at 100V for 1 hour. Of all samples 10 μL was loaded with 2 μL loadingbuffer. 5 μL was loaded of marker]] | ||
| + | |||
| + | Lane description: | ||
| + | {|style="color:black; background-color:white;" cellpadding="5" cellspacing="0" border="1" | ||
| + | |'''#''' | ||
| + | |'''Description''' | ||
| + | |'''Expected Length (bp)''' | ||
| + | |'''Status''' | ||
| + | |'''Remarks''' | ||
| + | |- | ||
| + | |1 | ||
| + | |marker | ||
| + | |n/a | ||
| + | |n/a | ||
| + | |n/a | ||
| + | |- | ||
| + | |2 | ||
| + | |Digested K398328T | ||
| + | |252, 1647, 69, 36, 3246 | ||
| + | |✗ | ||
| + | | | ||
| + | |- | ||
| + | |3 | ||
| + | |Digested K398407T | ||
| + | |2552 | ||
| + | |✗ | ||
| + | | | ||
| + | |- | ||
| + | |4 | ||
| + | |pSB1T3 with B0015 (control) | ||
| + | |172, 2463 | ||
| + | |✗ | ||
| + | |The upper band is most likely circular pSB1T3. There's a strange band around 600 bp. | ||
| + | |- | ||
| + | |5 | ||
| + | |Digested K398000C | ||
| + | |654, 252, 1647, 69, 36, 2004 | ||
| + | |✗ | ||
| + | | | ||
| + | |- | ||
| + | |6 | ||
| + | |Digested K398000C | ||
| + | |495, 2901 | ||
| + | |? | ||
| + | |There's no band of 495 bp | ||
| + | |- | ||
| + | |7 | ||
| + | |Digested K398002C | ||
| + | |2243 | ||
| + | |✓ | ||
| + | | | ||
| + | |- | ||
| + | |8 | ||
| + | |Digested K398200C | ||
| + | |3120 | ||
| + | |✗ | ||
| + | | | ||
| + | |- | ||
| + | |9 | ||
| + | |Digested K398201C | ||
| + | |558, 135, 177, 1887 | ||
| + | |✓ | ||
| + | | | ||
| + | |- | ||
| + | |10 | ||
| + | |Digested K398400C | ||
| + | |2529 | ||
| + | |✗ | ||
| + | | | ||
| + | |- | ||
| + | |11 | ||
| + | |pSB1T3 (control) | ||
| + | |2463 | ||
| + | |? | ||
| + | |A bright band around 2500 bp was shown. There are also a lot of unexplainable bands visible. | ||
| + | |} | ||
| + | |||
| + | Unfortunately most of the lanes showed incorrect bands. So from this gel we conclude that the ligation has failed. | ||
Latest revision as of 21:07, 16 August 2010
Contents |
Lab work
Ordered DNA
The transformations of 19 July containing the different ligations gave colonies (~5 per plate). We picked 5 colonies per plate and performed a colony PCR.
Alkane degradation
Unfortunately there were no transformants on yesterday's plates. This is most likely due to the fact that the ligation didn't work with the small pieces of DNA of the RBSs. Next plan is to cut open the RBS plasmid without removing the RBS (with SpeI and PstI) and insert the gene in this plasmid. We will try this tomorrow.
Salt Tolerance
The overnight ligation was transformed using the standard method and grown up overnight at 37 degrees C.
Emulsifier
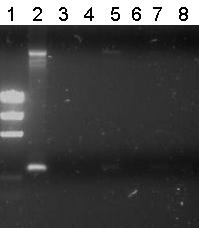
Lane Description
| # | Description | Expected lenght (bp) | Primer | Status |
| M1 | EZ Ladder | n/a | n/a | n/a |
| 1 | B0032 (control) | 220 | G00100 + G00101 | ✓ |
| 2 | Transformant #1 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 3 | Transformant #2 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 4 | Transformant #3 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 5 | Transformant #4 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 6 | Transformant #5 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 7 | Transformant #6 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
| 8 | Transformant #7 of ligation mix R0011-B0032 | 300 | G00100 + G00101 | ✗ |
Unfortunately all other lanes were empty or the same as the negative control. So from this gel we conclude that the ligation has failed.
Characterisation of Anderson RBS sequences
Assembly of reference construct
Method 1
Hypothesis II The transformants that were replated on kanamycin plates produced colonies, indicating that hypothesis II was invalid. To test whether these colonies did contain the proper insert however, a colony PCR was performed:
Lane description:
| # | Description | Expected Length (bp) | Primers | Status | Remarks |
| 1 | SmartLadder | n/a | n/a | ✓ | |
| 2 | PCR product of I13401 | 1239 | G00101 + G00101 | ✓ | |
| 3 | XbaI and PstI digested I13401 | 1239 | None | ✓ | No additional bands visible (see hypothesis I) |
| 4 | Transformant #1 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 5 | Transformant #4 of ligation mix: E-K081005-S + E-pSB1AK2-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 6 | Transformant #2 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 7 | Transformant #3 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 8 | Transformant #4 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 9 | Transformant #5 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 10 | transformant #6 of ligation mix: E-K081005-S + E-pSB1AK3-I13401-X | 1239 | G00101 + G00101 | ✗ | |
| 11 | Transformant #1 of ligation mix: E-pSB1AK3-I13401-X (ligation control) | 1173 | G00101 + G00101 | ✗ | |
| 12 | Transformant #2 of ligation mix: E-pSB1AK3-I13401-X (ligation control) | 1173 | G00101 + G00101 | ✗ | |
| 13 | Transformant #3 of ligation mix: E-pSB1AK3-I13401-X (ligation control) | 1173 | G00101 + G00101 | ✗ | |
| 14 | transformant #1 of digestion mix: E-pSB1AK3-I13401-X (digestion control) | 1239 | G00101 + G00101 | ✗ | |
| 15 | transformant #11 of ligation mix: E-pSB1AK3-I13401-X (digestion control) | 1239 | G00101 + G00101 | ✗ | |
| 16 | I13600 in pSB1A2 transformant #1 | G00101 + G00101 | ✗ | ||
| 17 | I13600 in pSB1A2 transformant #2 | G00101 + G00101 | ✗ | ||
| 18 | I13600 in pSB1A2 transformant #3 | G00101 + G00101 | ✗ | ||
| 19 | BioRad EZ Load | N/A | N/A | ✓ |
Method 3
TOP10 competent cells were transformed with yesterday's ligation product.
Method 4
The obtained colonies on ampicillin plates were screened for GFP fluorescence. No discernable fluorescence was seen under UV excitation. It was decided to perform a single colony PCR on 17 randomly selected colonies to check for the proper insert:
Lane description:
| # | Description | Expected Length (bp) | Primers | Status | Remarks |
| 1 | SmartLadder | Varies | n/a | ✓ | |
| 2 | PCR product of E0240 | 1114 | G00100 + G00101 | ? | |
| 3 | transformant #1 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 4 | transformant #2 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 5 | transformant #3 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 6 | transformant #4 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 7 | transformant #5 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 8 | transformant #6 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 9 | transformant #7 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 10 | transformant #8 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 11 | transformant #9 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 12 | transformant #10 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 13 | transformant #11 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 14 | transformant #12 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 15 | transformant #13 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 16 | transformant #14 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 17 | transformant #15 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 18 | transformant #16 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 19 | transformant #17 of ligation mix: S-J23100-pSB1A2-P + X-E0240-P | 1157 | G00100 + G00101 | ? | |
| 20 | BioRad EZ Load | Varies | N/A | ✓ |
The 43 bp difference was not discernable from gel, and thus this gel is quite inconclusive concerning the presence of positive colonies.
Solvent Tolerance and Hydrocarbon Sensing
Characteristic digestion of isolated plasmids
To determine whether the plasmids are correct. Characteristic cuts were made using the restriction enzyme PvuI.
| # | Sample | Enzyme 1 | Buffer | Needed fragments |
| 1 | 1450 μg K398328T | PvuII | Buffer II | 252, 1647, 69, 36, 3246 |
| 2 | 378 μg K398407T | PvuII | Buffer II | 2552 |
| 3 | 716.5 μg Control (pSB1T3, B0015: this does not ligate) | PvuII (this enzyme does not work here) | Buffer II | 172, 2463 |
| 4 | 245.4 μg K398300C | PvuII | Buffer II | 654, 252, 1647, 69, 36, 2004 |
| 5 | 1481.4 μg K398000C | PvuII | Buffer II | 495, 2901 |
| 6 | 1198.5 μg K398002C | PvuII | Buffer II | 2243 |
| 7 | 42.3 μg K398002C | PvuII | Buffer II | 3120 |
| 8 | 1612.8 μg K398200C | PvuII | Buffer II | 558, 135, 177, 1887 |
| 9 | 1633.3 μg K398201C | PvuII | Buffer II | 2529 |
| 10 | 938.6 μg K398400C | PvuII | Buffer II |
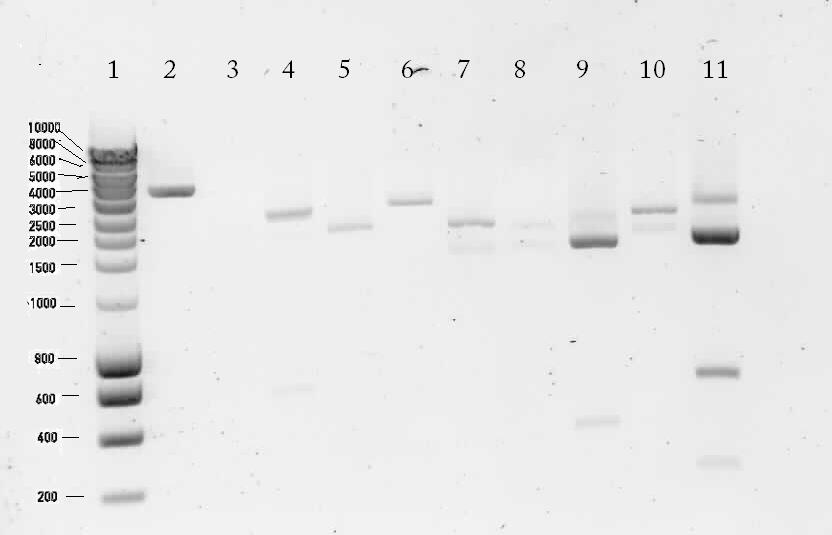
Results of the digestion on 1% agarose gel
Lane description:
| # | Description | Expected Length (bp) | Status | Remarks |
| 1 | marker | n/a | n/a | n/a |
| 2 | Digested K398328T | 252, 1647, 69, 36, 3246 | ✗ | |
| 3 | Digested K398407T | 2552 | ✗ | |
| 4 | pSB1T3 with B0015 (control) | 172, 2463 | ✗ | The upper band is most likely circular pSB1T3. There's a strange band around 600 bp. |
| 5 | Digested K398000C | 654, 252, 1647, 69, 36, 2004 | ✗ | |
| 6 | Digested K398000C | 495, 2901 | ? | There's no band of 495 bp |
| 7 | Digested K398002C | 2243 | ✓ | |
| 8 | Digested K398200C | 3120 | ✗ | |
| 9 | Digested K398201C | 558, 135, 177, 1887 | ✓ | |
| 10 | Digested K398400C | 2529 | ✗ | |
| 11 | pSB1T3 (control) | 2463 | ? | A bright band around 2500 bp was shown. There are also a lot of unexplainable bands visible. |
Unfortunately most of the lanes showed incorrect bands. So from this gel we conclude that the ligation has failed.
 "
"