Team:Stanford/Notebook/Lab Work/Week 3
From 2010.igem.org
(→Francisco's Notebook) |
(→Francisco's Notebook) |
||
| Line 133: | Line 133: | ||
*Mini-prepped GFP, RFP and terminator plasmids. Mini-prep results: | *Mini-prepped GFP, RFP and terminator plasmids. Mini-prep results: | ||
| - | {| | + | {| border = "1" |
! Part !! 260/280 !! 260/230 !! ng/uL | ! Part !! 260/280 !! 260/230 !! ng/uL | ||
|- | |- | ||
Revision as of 00:02, 29 July 2010

| Home | Project | Applications | Modeling | Parts | Team | Notebook |
Spring: Brainstorming | Spring Meetings
Summer: Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Summaries
|
7/12 Monday
Christopher's Notebook
- Run Gel of Restricted Digested RBS+GFP+Terminators (I746908 partial)
- Gel Extract
- Run Gel of Gel Extracted Digest Products from Friday:
3C5 E/P 2738 bp (buffer 3) F2620 E/S (on pSB1A2) 1061 bp
- I746908 E/P (on psb1A2) 2093 bp-can’t separate
- B0034 X/P (on psb1A2) 12 bp-do NOT run on gel
- J23100 E/S (on psb1A2) 35 bp-do NOT run on gel
Karina's Notebook
Goal: We couldn't extract the terminators because they were too small and ran off the gel. After discussing with Laura and Francisco, we decided to clone GFP and RFP into the plasmid in which the terminators come (pSB1AK3).
Make BSA
- BSA comes as 100x
- made 1 mL of 1x solution by adding 900uL H20 and 100 uL BSA
Cut the GFP and RFP linearized plasmid (prev. cut at S and P) at EcoRI restriction site
- used recipe as described on July 9, 2010
- only used 1 enzyme, therefore used 27 uL H20
- put in waterbath at 10:30 am, and plan to take out after lunch.
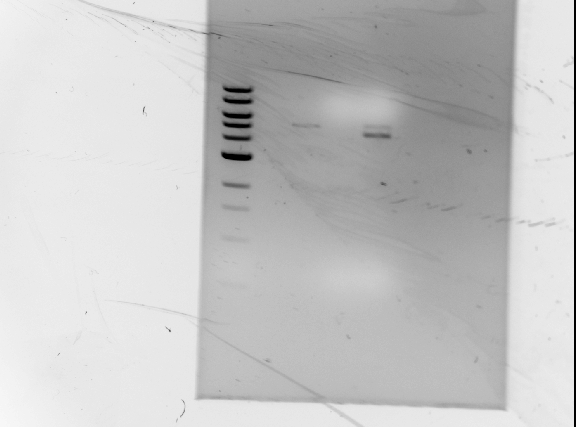
Results of diagnostic gel
Observations/Troubleshooting:
- no DNA bands for GFP
- not enough DNA?
- will double DNA concentration during digests
- not enough DNA?
- bands are too large to be RFP
- possible partial digest?
- leave digests overnight
Digests
Digest miniprepped GFP, RFP and Terminators.
- Cut GFP and RFP at E and S
- Cut terminators at E and X
Recipe
- 25 uL DNA
- 1.0 uL enzyme 1
- 1.0 uL enzyme 2
- 5.0 uL NEB Buffer 2
- 5.0 uL BSA (10x)
- 13 uL H20
Total Volume: 50 uL
We used twice as much DNA than the usual protocol because our DNA concentrations from the minipreps were very low.
Prepared 3 reactions for each RSID; ran 6 PCR reactions total.
Ran digests overnight
Francisco's Notebook
- Reran the diagnostic gel (see Karina's Notebook):
- Lessons learned:
- When loading small volumes, use narrow wells.
- When suspecting there are no bands, increase the exposure time to confirm.
Greg's Notebook
- Ran gel with pSB3c5, F2620, I746908
- I746908 didn't work, extracted pSB3c5 and F2620
- Worked on MDV powerpoint presentation
- Ran diagnostic gel of gel extraction results
- pSB3c5 worked, F2620 left only a faint line
- With Alex, miniprepped pSB1k3, I746908, I0500
- Performed nanodrop on miniprep:
| part # | 280 | 230 | concentration (ng/uL) |
| I0500r | 1.87 | 1.28 | 62.5 |
| pSB1k3 | 1.81 | 1.14 | 67.6 |
| I746908 | 1.89 | 1.73 | 175.7 |
- Digested more F2620, pSB3c5, and I0500. Also digested 3 superfolded GFP inserts
7/13 Tuesday
Christopher's Notebook
- Run Gels (0.8%) of Restriction Digests:
1M, 2M, 2J (X/P) F2620 (E/S) 3C5 (E/P) I0500 (E/S)
Note: for PCR of I746908: after first gel of PCR product, gel extract, and then do a restriction digest. After restriction digest, do a reaction cleanup; pieces are ready for ligation.
Laura's Notebook
- restreaked plates from 7/1/10 (with Karina)
- for each plate, pick 8 colonies and streak, starting at the center of the "pie piece," and working towards the perimeter of the plate
- plate ID's:
Greg's Notebook
- Made gel, ran digests from yesterday
- Extracted pBAD (I0500) and pSB3c5
- Practiced primer design
- Inventoried my box
7/14 Wednesday
Laura's Notebook
- plates restreaked yesterday look good- bacteria grew well, some individual colonies
- helped Francisco with minipreps (Promega kit) of : terminator, GFP, RFP
- Francisco set up liquid cultures yesterday afternoon
- Francisco poured, ran 0.8% gel
- yesterday's digestions, with today's minipreps as controls
- gel extraction: (I cut out bands, Francisco and Karina extracted)
| part | tube mass (g) | tube + gel (g) | gel (g) | volume QG (uL) |
| terminator | 1.03 | 1.14 | 0.11 | 330 |
| RFP | 1.11 | 1.18 | 0.07 | 210 |
| GFP | 1.03 | 1.13 | 0.10 | 300 |
Francisco's Notebook
- We've decided to cut out GFP and RFP inserts and ligate them into a linearized terminator plasmid. GFP and RFP inserts are fairly large (~700 bp) and would be easier to gel extract than a terminator insert. The terminator we are using is B1006.
- Mini-prepped GFP, RFP and terminator plasmids. Mini-prep results:
| Part | 260/280 | 260/230 | ng/uL |
|---|---|---|---|
| Term | 1.84 | 0.87 | 62.9 |
| RFP | 1.87 | 1.65 | 235.5 |
| GFP | 1.85 | 1.05 | 121.7 |
- Digested RFP and GFP plasmids with EcoR1 and Spe1; terminator plamids with EcoR1 and Xba1.
- Gel extracted the RFP and GFP inserts and the linearized terminator plasmid.
Greg's Notebook
- Worked on MDV presentation
7/16 Friday
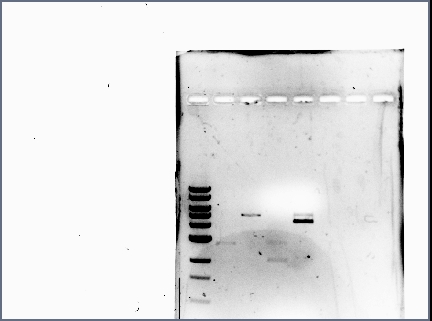
Francisco's Notebook
- Ran a diagnostic gel of the digestion done on Wednesday
- GFP and RFP plasmids (cut at E, S); Term plasmid (cut at E, X):
- Results: the terminator backbone has a bright band, but the gel extracted GFP and RFP inserts had very faint bands (low concentrations). When we ligate, we may need to use larger volumes of the inserts.
 "
"