Team:Lethbridge/Notebook/Lab Work/July
From 2010.igem.org
JustinVigar (Talk | contribs) (→July 5/2010) |
JustinVigar (Talk | contribs) (→July 5/2010) |
||
| Line 95: | Line 95: | ||
<tr><td>15</td><td>33 - lumazine (G9)</td><td>1 DNA + 2 Dye (6X) + 7 Milli-Q H<sub>2</sub>O</td></tr> | <tr><td>15</td><td>33 - lumazine (G9)</td><td>1 DNA + 2 Dye (6X) + 7 Milli-Q H<sub>2</sub>O</td></tr> | ||
</table><br> | </table><br> | ||
| + | |||
| + | Ran gel at 100V for 45 minutes.<br> | ||
| + | |||
| + | ==July 5/2010 Evening== | ||
| + | |||
| + | <b>Objective:</b>To over-express CFP complete in DH5α<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | |||
| + | 1) Inoculated 6mL culture with ampicillin and glycerol stock A6-May 13,2010 CFP complete | ||
| + | 2) Went in the shaker at 6:50pm | ||
| + | |||
| + | ==July 6/2010== | ||
| + | (In lab: JV, AV, HB)<br> | ||
| + | <b>Objective:</b>To continue the over-expression of CFP complete in DH5α<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | 1) Put both cultures (taken out of shaker at 9:15am) and put them in 500mL of LB w/ Amp.<br> | ||
| + | 2) initial OD was 0.071 (600λ)<br> | ||
| + | |||
| + | Issue:<br> | ||
| + | *After checking the the sequencing it was evident that our promotor is always off. It is turned off by the product of the gene TetR. Which is not part of our construct. <br> | ||
| + | |||
| + | table><table border ="3"> | ||
| + | <tr><td><b>Time (hours)</b></td>OD (600λ)</tr> | ||
| + | <tr><td>0</td><td>0.071</td></tr> | ||
| + | <tr><td>1</td><td>0.390</td></tr> | ||
| + | <tr><td>1.5(T<sub>0</sub>)</td><td>0.606</td></tr> | ||
| + | <tr><td>2.5 (T<sub>1</sub>)</td><td>1.250</td></tr> | ||
| + | <tr><td>3.5(T<sub>2</sub>)</td><td>3.04</td></tr> | ||
| + | <tr><td>4.5(T<sub>3</sub>)</td><td>2.75</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | <b>Results:</b>Ran samples on a 15% SDS-page. The gel did not show any signs of over-expression.<br> | ||
==June 2/2010== | ==June 2/2010== | ||
Revision as of 23:04, 19 July 2010
Back to Notebook
Back to Lab Work
Contents |
July 2010
July 5/2010
(In Lab: JV, AV, HB)
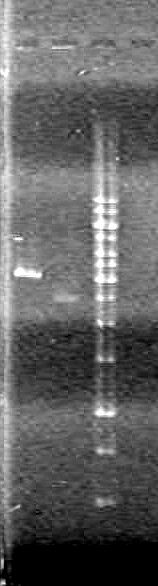
Objective: Run a 1% agarose gel of purified PCR samples from June 24/10
Method:
| Lane | Sample | Components (µL) |
| 1 | 1kb Ladder | 0.5 Ladder + 2 Dye (6X) + 7.5 Milli-Q H2O |
| 2 | 1 - pBAD (A4) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 3 | 2 - pBAD (A5) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 4 | 3 - SRBS (A6) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 5 | 4 - SRBS (A7) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 6 | 5 - CFP Complete (A8) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 7 | 6 - SRBS (A10) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 8 | 7 - EYFP (B1) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 9 | 8 - N term tag (B2) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 10 | 9 - pSB NEYFP (B4) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 11 | 10 - pSB NEYFP (B5) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 12 | 11 - CFP (B6) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 13 | 12 - pBAD-TetR (B10) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 14 | 13 - D3 | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 15 | 14 - C term (D4) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 16 | 15 - C term (D5) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 17 | 16 - pLacI (D6) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 18 | 17 - NEYFP (E2) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 19 | 18 - CEYFP (E6) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 20 | 19 - CEYFP (E7) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 1 | 1kb ladder | 0.5 Ladder + 2 Dye (6X) + 7.5 Milli-Q H2O |
| 2 | 20 - EYFP (E8) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 3 | 21 - EYFP (E9) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 4 | 22 - EYFP (E10) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 5 | 23 - ECFP (F1) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 6 | 24 - ECFP (F2) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 7 | 25 - ECFP (F3) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 8 | 26 - pBAD-TetR (F4) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 9 | 27 - pBAD-TetR (F5) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 10 | 28 - EYFP (G1) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 11 | 29 - pSB CEYFP (G4) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 12 | 30 - pBAD (1) (G6) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 13 | 31 - pBAD (2) (G7) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 14 | 32 - N term tag (G8) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
| 15 | 33 - lumazine (G9) | 1 DNA + 2 Dye (6X) + 7 Milli-Q H2O |
Ran gel at 100V for 45 minutes.
July 5/2010 Evening
Objective:To over-express CFP complete in DH5α
Method:
1) Inoculated 6mL culture with ampicillin and glycerol stock A6-May 13,2010 CFP complete 2) Went in the shaker at 6:50pm
July 6/2010
(In lab: JV, AV, HB)
Objective:To continue the over-expression of CFP complete in DH5α
Method:
1) Put both cultures (taken out of shaker at 9:15am) and put them in 500mL of LB w/ Amp.
2) initial OD was 0.071 (600λ)
Issue:
- After checking the the sequencing it was evident that our promotor is always off. It is turned off by the product of the gene TetR. Which is not part of our construct.
| Time (hours) | OD (600λ)|
| 0 | 0.071 |
| 1 | 0.390 |
| 1.5(T0) | 0.606 |
| 2.5 (T1) | 1.250 |
| 3.5(T2) | 3.04 |
| 4.5(T3) | 2.75 |
Results:Ran samples on a 15% SDS-page. The gel did not show any signs of over-expression.
June 2/2010
(In Lab: JV)
Objective: Isolate plasmid DNA of RBS-xylE (BBa_J33204) from DH5α cells and confirm results.
Method: "Mini-prep" the plasmid DNA using boiling lysis miniprep. Then restrict the DNA once and run on a 1% agarose gel (TAE).
Restriction Reaction
| Ingredient | Volume(µL) |
| MilliQ H20 Water | 15.75 |
| Orange Buffer (10x) | 2 |
| pDNA (rbs-xylE) | 2 |
| EcoRI | 0.25 |
Unrestricted Control
| Ingredient | Volume(µL) |
| MilliQ H20 Water | 16 |
| Orange Buffer (10x) | 2 |
| pDNA (rbs-xylE) | 2 |
DNA was restricted for 80 minutes at 37oC.
Analyzed results on a 1% agarose gel. Load order as follows:
| Lane | Sample | Volume Sample (µL) | Volume Loading Dye (µL) |
| 1 | Restricted RBS-xylE | 10 | 2 |
| 1 | Unestricted RBS-xylE† | 1 | 2 |
| 1 | 1kb Ladder†† | 2 | 2 |
† Added 9µL MilliQ H2O
†† Added 8µL MilliQ H2O
Ran gel at 100V from 2 hours.
Results:
Conclusions: Plasmid DNA prep and restriction was successful.
Objective: Ligate rbs-xylE (Bba_J33204) to our double terminator, and insert it into the pSB1T3 plasmid backbone.
Method:
- Restrictions
- Restrict rbs-xylE wit EcoRI and SpeI (Red Buffer)
- Restrict the double terminator with XbaI and PstI (Tango Buffer)
- Restrict pSB1T3 with EcoRI and PstI (Red Buffer)
Component Volume (µL) MilliQ H2O 15.5 Buffer 2 pDNA 2 Enzyme 0.25 + 0.25 Set up control reaction as follows:
- MilliQ H2O - 16µL
- Buffer - 2µL
- pDNA - 2µL
Incubated reactions for 65 minutes at 37oC
Killed enzymes by incubating reactions for 10 minutes at 65oC
- Ligation
Reaction set up as follows:- T4 DNA ligase - 0.25µL
- rbs-xylE - 5µL
- dT - 3µL
- pSB1T3 - 8µL
- 10x Ligation Buffer - 2µL
- MilliQ H2O - 1.75µL
Killed enzymes by incubating reactions for 10 minutes at 80oC</ul>June 2/2010 - Evening
Objective: Set up new ligations of pLacI and sRBS-Lum-dT according to Tom Knight's protocol. Previous ligation had very little DNA.
Relevant Information:
- Want a final mass of 25ng of each pDNA in the ligation mix.
- Final concentration of pDNA in restriction digest should be 25-50ng/µL.
- Tom Knight's restriction reaction is 50µL, therefore there should be 1000ng pDNA in each restriction digest.
- Identified the following plasmids in our working plasmids box:
Common Name Location Concentration (ng/µL) Volume/rxn (µL) pLacI Maxiprep A9 990 ~1 pLacI (B1) A6 440 ~2 sRBS-Lum-dT (2) A1 965 ~1 sRBS-Lum-dT (1) A2 1145 ~1 sRBS-Lum-dT Maxiprep B8 4780 ~.2 sRBS-Lum-dT B7 4375 ~.25 sRBS-Lum-dT (1) G2 335 ~3 sRBS-Lum-dT (2) G3 965 ~2 - Make a 1:10 dilution of sRBS-Lum-dt maxiprep (D8) and sRBS-Lum-dT (B7). 0.5µL pDNA in 4.5µL water.
- Cut pLacI with EcoRI and SpeI
- Cut sRBS-Lum-dT with XbaI and PstI
- Cut pSB1T3 with EcoRI and PstI
- Will have total of 12 ligation reactions, want 12x2µL of pSB1T3 to add to each, therefore want 25µL of pSB1T3.
Method:
Restriction
Name [pDNA] (ng/µL) Volume
pDNA (µL)Volume
Water (µL)Volume
Buffer (µL)Enzymes Total Volume sRBS-Lum-dT (A1) 965 1 43.5 5 0.25µL XbaI
0.25µL PstI50 sRBS-Lum-dT (A2) 1145 1 43.5 5 0.25µL XbaI
0.25µL PstI50 pLacI Maxiprep (A1) 990 1 43.5 5 0.25µL EcoRI
0.25µL SpeI50 sRBS-Lum-dT Maxiprep(B8) 4780 2 (of 1:10 dilution) 42.5 5 0.25µL XbaI
0.25µL PstI50 sRBS-Lum-dT (B7) 4375 2.5 (of 1:10 dilution) 42 5 0.25µL XbaI
0.25µL PstI50 pLacI (D6) 440 2 42.5 5 0.25µL EcoRI
0.25µL SpeI50 sRBS-Lum-dT (G2) 335 3 41.5 5 0.25µL XbaI
0.25µL PstI50 sRBS-Lum-dT (G3) 540 2 42.5 5 0.25µL XbaI
0.25µL PstI50 pSB1T3 25 12.5 7 5 0.25µL EcoRI
0.25µL PstI50 Incubate for 30 minutes at 37oC (Start- 12:10pm; End- 12:40pm)
Heat kill enzymes at 80oC for 20 minutes
Ligation:
In a 10µL final volume, add:- 2µL of sRBS-Lum-dT component
- 2µL of pLacI component
- 2µL of pSB1T3 component
- 1µL of T4 Buffer
- 0.25µL of T4 DNA Ligase
- 2.75µL of MilliQ H2O
Incubate for 30 minutes at room temperature to ligate
Incubate for 20 minutes at 80oC to heat kill
- Restrict rbs-xylE wit EcoRI and SpeI (Red Buffer)
 "
"


 ]
]
 ]
]
 ]
]
 ]
]
 ]
]
 ]
]
 ]
]
 ]
]