Team:Heidelberg/Notebook/BSDesign/July
From 2010.igem.org
(→raPCR for the construction of anti miRsAg-shRNA sponges) |
|||
| (12 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{:Team:Heidelberg/Single_Pagetop|note_BSDesign}} | {{:Team:Heidelberg/Single_Pagetop|note_BSDesign}} | ||
{{:Team:Heidelberg/Side_Top}} | {{:Team:Heidelberg/Side_Top}} | ||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#4e93a4; border:1.53px solid #333333;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#c85000;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July<font color="white">July</font>] | ||
| + | |- style="background:#c85000; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="3"| ||'''1'''||'''2'''||'''3'''||'''4''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 5]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 6]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 7]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 8]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 10]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#05.2F07.2F2010_-_11.2F07.2F2010 11]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 12]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 13]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 14]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 15]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 16]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 17]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 18]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#12.2F07.2F2010_-_19.2F07.2F2010 19]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#20.2F07.2F2010 20]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#21.2F07.2F2010 21]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#22.2F07.2F2010 22]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#23.2F07.2F2010 23]'''||'''24'''||'''25''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#26.2F07.2F2010 26]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#27.2F07.2F2010 27]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#28.2F07.2F2010 28]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#29.2F07.2F2010 29]'''||'''30'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/July#31.2F07.2F2010 31]'''|| | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | | colspan="7"| | ||
| + | <span style="color:#ffffff">-</span> | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#f09600; border: 1.5px solid #000000;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#f09600;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August<font color="white">August</font>] | ||
| + | |- style="background:#f09600; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="6"| ||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#01.2F08.2F2010 1]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#02.2F08.2F2010 2]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#03.2F08.2F2010 3]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#04.2F08.2F2010 4]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#05.2F08.2F2010 5]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#06.2F08.2F2010 6]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#07.2F08.2F2010 7]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#08.2F08.2F2010 8]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#09.2F08.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#10.2F08.2F2010 10]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/August#11.2F08.2F2010 11]'''||'''12'''||'''13'''||'''14'''||'''15''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''16'''||'''17'''||'''18'''||'''19'''||'''20'''||'''21'''||'''22''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''23'''||'''24'''||'''25'''||'''26'''||'''27'''||'''28'''||'''29''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''30'''||'''31'''||colspan="5"| | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#f09600; border: 1.5px solid #333333;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#009be1;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September<font color="#ffecba">September</font>] | ||
| + | |- style="background:#009be1; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="2"| ||'''1'''||'''2'''||'''3'''||'''4'''||'''5''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''6'''||'''7'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#08.2F09.2F2010 8]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#09.2F09.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#10.2F09.2F2010 10]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#11.2F09.2F2010 11]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#12.2F09.2F2010 12]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#13.2F09.2F2010 13]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#14.2F09.2F2010 14]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#15.2F09.2F2010 15]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#16.2F09.2F2010 16]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#17.2F09.2F2010 17]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#18.2F09.2F2010 18]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#19.2F09.2F2010 19]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#20.2F09.2F2010 20]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#21.2F09.2F2010 21]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#22.2F09.2F2010 22]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#23.2F09.2F2010 23]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#24.2F09.2F2010 24]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#25.2F09.2F2010 25]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#26.2F09.2F2010 26]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#27.2F09.2F2010 27]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#28.2F09.2F2010 28]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#29.2F09.2F2010 29]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/September#30.2F30.2F2010 30]'''||colspan="5"| | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="7"| | ||
| + | <span style="color:#ffffff">-</span> | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| cellpadding="5" cellspacing="0" align="center" style="text-align: center; color:#f09600; border: 1.5px solid #333333;" | ||
| + | |- border="0" | ||
| + | ! colspan="7" style="background:#78b41e;" | [https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October<font color="white">October</font>] | ||
| + | |- style="background:#78b41e; color:white" | ||
| + | |width="20pt"|'''M'''||width="20pt"|'''T'''||width="20pt"|'''W'''||width="20pt"|'''T'''||width="20pt"|'''F'''||width="20pt"|'''S'''||width="20pt"|'''S''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="4"| ||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#01.2F10.2F2010 1]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#02.2F10.2F2010 2]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#03.2F10.2F2010 3]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#04.2F10.2F2010 4]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#05.2F10.2F2010 5]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#06.2F10.2F2010 6]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#07.2F10.2F2010 7]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#08.2F10.2F2010 8]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#09.2F10.2F2010 9]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#10.2F10.2F2010 10]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#11.2F10.2F2010 11]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#12.2F10.2F2010 12]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#13.2F10.2F2010 13]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#14.2F10.2F2010 14]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#15.2F10.2F2010 15]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#16.2F10.2F2010 16]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#17.2F10.2F2010 17]''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#18.2F10.2F2010 18]'''|||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#19.2F10.2F2010 19]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#20.2F10.2F2010 20]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#21.2F10.2F2010 21]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#22.2F10.2F2010 22]'''||'''[https://2010.igem.org/Team:Heidelberg/Notebook/BSDesign/October#23.2F10.2F2010 23]'''||'''24''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |'''25'''||'''26'''||'''27'''||'''28'''||'''29'''||'''30'''||'''31''' | ||
| + | |- style="background:#f2f2f2; color:#f09600" | ||
| + | |colspan="7"| | ||
| + | <span style="color:#ffffff">-</span> | ||
| + | |} | ||
{{:Team:Heidelberg/Side_Bottom}} | {{:Team:Heidelberg/Side_Bottom}} | ||
| Line 10: | Line 92: | ||
== 05/07/2010 - 11/07/2010 == | == 05/07/2010 - 11/07/2010 == | ||
<br /> | <br /> | ||
| - | === Preperation of competent E. coli Top10 and DH5alpha === | + | === Preperation of chemically competent cells E. coli Top10 and DH5alpha === |
* plating of E. coli Top10 and DH5alpha on a agar plate (LB, without Amp); Preperation of competent cells according to the following protocol: | * plating of E. coli Top10 and DH5alpha on a agar plate (LB, without Amp); Preperation of competent cells according to the following protocol: | ||
First, a 20 ml over night culture was inoculated in antibiotic free LB medium from a fresh single colony and transferred into 400 ml antibiotic free LB medium the next day. This culture was incubated at 37 °C while shacking until an OD600 of 0.5 – 0.6 was achieved. The culture was than cooled down on ice, centrifuged (8 min, 4 °C, 3500 rpm), the supernatant discarded and the pellet resuspended in 10 ml 100 mM CaCl2. After addition of further 190 ml 100 mM CaCl2 the suspension was incubated on ice for 30 min. The suspension was than again centrifuged (8 min, 4 °C, 3500 rpm), the supernatant discarded, the pellet resuspended in 20 ml 82.5 mM CaCl2 with 17.5 % glycerol and aliquoted. The aliquots were flash frozen in liquid nitrogen and than stored at -80 °C until usage. | First, a 20 ml over night culture was inoculated in antibiotic free LB medium from a fresh single colony and transferred into 400 ml antibiotic free LB medium the next day. This culture was incubated at 37 °C while shacking until an OD600 of 0.5 – 0.6 was achieved. The culture was than cooled down on ice, centrifuged (8 min, 4 °C, 3500 rpm), the supernatant discarded and the pellet resuspended in 10 ml 100 mM CaCl2. After addition of further 190 ml 100 mM CaCl2 the suspension was incubated on ice for 30 min. The suspension was than again centrifuged (8 min, 4 °C, 3500 rpm), the supernatant discarded, the pellet resuspended in 20 ml 82.5 mM CaCl2 with 17.5 % glycerol and aliquoted. The aliquots were flash frozen in liquid nitrogen and than stored at -80 °C until usage. | ||
<br /> | <br /> | ||
| + | |||
=== Cell Culture Starting === | === Cell Culture Starting === | ||
* thawing of Huh-7, HeLa p4 and HEK-293 cells according to the following protocol | * thawing of Huh-7, HeLa p4 and HEK-293 cells according to the following protocol | ||
| Line 19: | Line 102: | ||
:* once, the probe was nearly completely thawn, cells were thrown into pre-warmed DMEM (10 % FCS, L-Glut, P/S) and gentely mixed | :* once, the probe was nearly completely thawn, cells were thrown into pre-warmed DMEM (10 % FCS, L-Glut, P/S) and gentely mixed | ||
:* cells were spinned down at 800 rpm, 3 min; supernatant was discarded | :* cells were spinned down at 800 rpm, 3 min; supernatant was discarded | ||
| - | :* the | + | :* the pellet was resuspended in 10 ml DMEM an plated on a p100 cell culture dish in the following media according to the different cell lines: |
<br /> | <br /> | ||
Media for Hela and Hek cells: | Media for Hela and Hek cells: | ||
| Line 35: | Line 118: | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
| + | ---- | ||
== 12/07/2010 - 19/07/2010 == | == 12/07/2010 - 19/07/2010 == | ||
| Line 60: | Line 144: | ||
=== design of measurement standard === | === design of measurement standard === | ||
<br /> | <br /> | ||
| - | * in order to design an appropriety standard respecting all the rules according to the standard of mammalian synthetic biology (RFC12), we developed a dual reporter | + | * in order to design an appropriety standard respecting all the rules according to the standard of mammalian synthetic biology (RFC12), we developed a dual reporter measurement construct with the following properties: |
:* bidirectional CMV promoter driving both reporter genes | :* bidirectional CMV promoter driving both reporter genes | ||
:* distabilized EGFP and EBFP2 as reporter genes, as they are really similar in their whole structure and amino-acid sequence | :* distabilized EGFP and EBFP2 as reporter genes, as they are really similar in their whole structure and amino-acid sequence | ||
| Line 68: | Line 152: | ||
:* vector backbone, containing an Amp resistance, Hygromycin resistance, pBR322 origin and an FRT site for stable integration | :* vector backbone, containing an Amp resistance, Hygromycin resistance, pBR322 origin and an FRT site for stable integration | ||
please, find more information about the measurement standard design in the measurment standard page | please, find more information about the measurement standard design in the measurment standard page | ||
| + | |||
| + | <br /><br /> | ||
| + | ---- | ||
== '''20/07/2010''' == | == '''20/07/2010''' == | ||
| Line 118: | Line 205: | ||
''' Gel Electrophoresis ''' | ''' Gel Electrophoresis ''' | ||
* PCR products from the 25 cycle PCR were analyzed on a Gel. Therefor, 5 ul of PCR product were mixed with 1 ul of 6x-loading dye and loaded on a 1.5 % agarose gel. The gel ran at 135 V for 55 min. | * PCR products from the 25 cycle PCR were analyzed on a Gel. Therefor, 5 ul of PCR product were mixed with 1 ul of 6x-loading dye and loaded on a 1.5 % agarose gel. The gel ran at 135 V for 55 min. | ||
| - | <br /> | + | <br /><br /> |
| + | ---- | ||
== ''' 21/07/2010 ''' == | == ''' 21/07/2010 ''' == | ||
| Line 190: | Line 278: | ||
''' Gel Electrophoresis ''' | ''' Gel Electrophoresis ''' | ||
* PCR products from the 25 cycle PCR were analyzed on a Gel. Therefor, 5 ul of PCR product were mixed with 1 ul of 6x-loading dye and loaded on a 1.5 % agarose gel. In addition, 2.5 ul of the 7-cycle PCR (07/21/2010) and 12-cycle PCR were loaded as well. The gel ran at 135 V for 55 min. | * PCR products from the 25 cycle PCR were analyzed on a Gel. Therefor, 5 ul of PCR product were mixed with 1 ul of 6x-loading dye and loaded on a 1.5 % agarose gel. In addition, 2.5 ul of the 7-cycle PCR (07/21/2010) and 12-cycle PCR were loaded as well. The gel ran at 135 V for 55 min. | ||
| - | <br /> | + | <br /><br /> |
| - | <br /> | + | <br /><br /> |
| + | |||
| + | ---- | ||
| + | |||
== 22/07/2010 == | == 22/07/2010 == | ||
<br /> | <br /> | ||
| Line 200: | Line 291: | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
| + | ---- | ||
| + | |||
== 23/07/2010 == | == 23/07/2010 == | ||
<br /> | <br /> | ||
| Line 205: | Line 298: | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
| + | ---- | ||
| + | |||
== 26/07/2010 == | == 26/07/2010 == | ||
<br /> | <br /> | ||
| Line 236: | Line 331: | ||
<br /> | <br /> | ||
| - | + | ||
| + | ---- | ||
== 27/07/2010 == | == 27/07/2010 == | ||
| Line 275: | Line 371: | ||
:set up the exact same reaction as on the day before, but using an anealing temperature of 65 °C | :set up the exact same reaction as on the day before, but using an anealing temperature of 65 °C | ||
*analysis of the result on a 2 % agarose gel, run 45 min @ 135 V | *analysis of the result on a 2 % agarose gel, run 45 min @ 135 V | ||
| + | <br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /><br /> | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
| - | + | ---- | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== 28/07/2010 == | == 28/07/2010 == | ||
| Line 314: | Line 397: | ||
<br /><br /> | <br /><br /> | ||
=== raPCR for the construction of anti miRsAg-shRNA sponges === | === raPCR for the construction of anti miRsAg-shRNA sponges === | ||
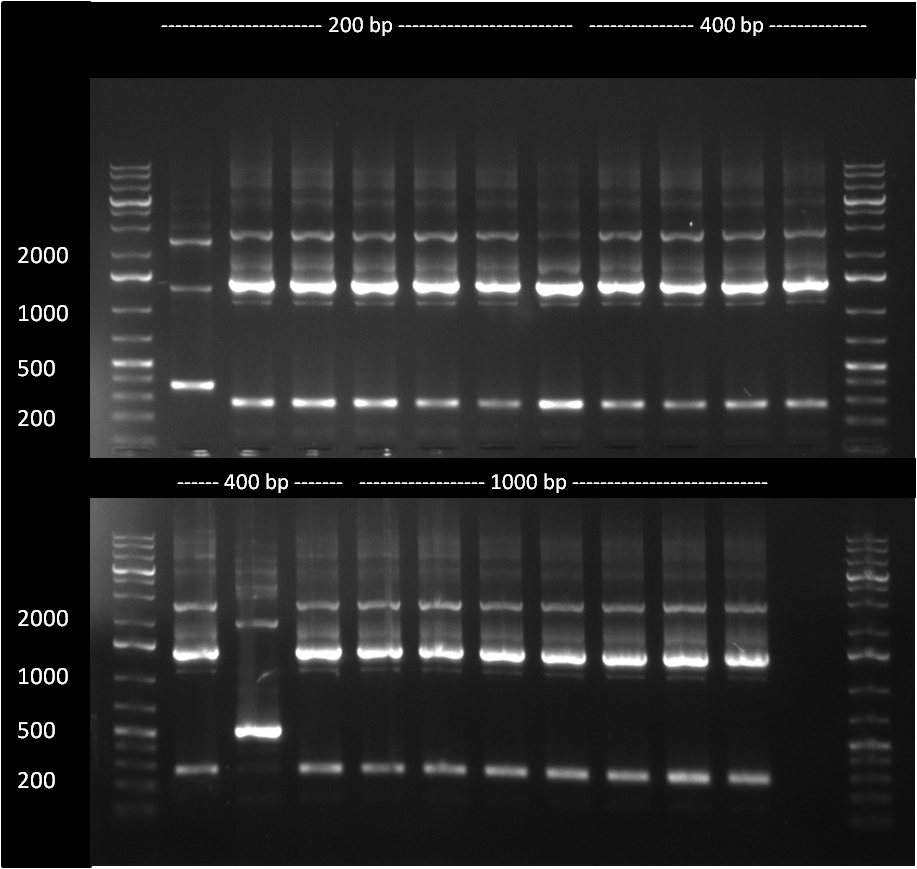
| - | [[Image:sponges1.png|thumb|750 px|In order to vary the length of the constructed sponges, three parameters were varied in the different PCRs: <br /> a) the stop oligos were either added before or after the 12 cycle PCR <br /> b) we used different amounts of binding site oligos (0.5, 1 or 1.5 ul each) <br /> c) different amounts of 12 cycle PCR product were used in the second, 25 cycle PCR (either 1 or 20 ul)]] | + | [[Image:sponges1.png|thumb|750 px|center|In order to vary the length of the constructed sponges, three parameters were varied in the different PCRs: <br /> a) the stop oligos were either added before or after the 12 cycle PCR <br /> b) we used different amounts of binding site oligos (0.5, 1 or 1.5 ul each) <br /> c) different amounts of 12 cycle PCR product were used in the second, 25 cycle PCR (either 1 or 20 ul)]] |
| - | + | ||
* the reaction was set up according to the standard miRACLE protocol; either 0.5, 1 or 1.5 ul of each binding site oligo were used. 0.5 ul were either added to the first and second PCR reaction or were not present in the first, but the second PCR. For analysis, 5 ul of each PCR product were loaded on a 1 % agarose gel, run @ 135 V for 45 min | * the reaction was set up according to the standard miRACLE protocol; either 0.5, 1 or 1.5 ul of each binding site oligo were used. 0.5 ul were either added to the first and second PCR reaction or were not present in the first, but the second PCR. For analysis, 5 ul of each PCR product were loaded on a 1 % agarose gel, run @ 135 V for 45 min | ||
Latest revision as of 03:17, 28 October 2010

Binding Site Design - July05/07/2010 - 11/07/2010
Preperation of chemically competent cells E. coli Top10 and DH5alpha
First, a 20 ml over night culture was inoculated in antibiotic free LB medium from a fresh single colony and transferred into 400 ml antibiotic free LB medium the next day. This culture was incubated at 37 °C while shacking until an OD600 of 0.5 – 0.6 was achieved. The culture was than cooled down on ice, centrifuged (8 min, 4 °C, 3500 rpm), the supernatant discarded and the pellet resuspended in 10 ml 100 mM CaCl2. After addition of further 190 ml 100 mM CaCl2 the suspension was incubated on ice for 30 min. The suspension was than again centrifuged (8 min, 4 °C, 3500 rpm), the supernatant discarded, the pellet resuspended in 20 ml 82.5 mM CaCl2 with 17.5 % glycerol and aliquoted. The aliquots were flash frozen in liquid nitrogen and than stored at -80 °C until usage.
Cell Culture Starting
Media for HUH-7 cells:
12/07/2010 - 19/07/2010
Preperation of RNA extracts for miRNA profiling
Protocol
design of diraPCR oligos
design of measurement standard
please, find more information about the measurement standard design in the measurment standard page
20/07/2010
................................................
................................................ (7x)
................................................
................................................
................................................ (25x)
................................................
................................................
21/07/2010
................................................
................................................ (12x)
................................................
................................................
................................................
................................................ (3x)
................................................
................................................
................................................ (25x)
................................................
................................................
22/07/2010
23/07/2010
26/07/2010
single binding site synthesis
PCR protocol:
................................................
................................................ (30x)
................................................
................................................
27/07/2010
................................................
................................................ (30x)
................................................
................................................
Single Binding Site Synthesis - Optimization
28/07/2010
raPCR for the construction of anti miRsAg-shRNA sponges In order to vary the length of the constructed sponges, three parameters were varied in the different PCRs: a) the stop oligos were either added before or after the 12 cycle PCR b) we used different amounts of binding site oligos (0.5, 1 or 1.5 ul each) c) different amounts of 12 cycle PCR product were used in the second, 25 cycle PCR (either 1 or 20 ul)
31/07/2010
diraPCR for constructing hsa-886-3p binding site patterns
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"