Team:Berkeley/Results
From 2010.igem.org
| (38 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
| - | + | == General Experimental Set Up== | |
| - | + | Our payload delivery assay involves the following steps: | |
# Feed the payload bacteria to the choanos and induce self-lysis with arabinose. Induction of self lysis and feeding are not necessarily at the same time. | # Feed the payload bacteria to the choanos and induce self-lysis with arabinose. Induction of self lysis and feeding are not necessarily at the same time. | ||
| - | # | + | # Use fluorescent microscopy to detect delivery of our GFP payload.<br> |
| - | + | ||
| - | + | ||
'''''Challenges''''' | '''''Challenges''''' | ||
| - | One of the challenges we faced in assaying for payload delivery was finding a media that the | + | One of the challenges we faced in assaying for payload delivery was finding a media that the Choanoflagellates liked and that accomodated lysis. After assaying several different media formulations, including CGM, LB, TB, ASW and four different mixtures of ASW and LB, we found that CGM media was the best media for Choano viability, E. Coli lysis, and protein delivery. For more details, see our characterization of the [https://2010.igem.org/Team:Berkeley/Project/Self_Lysis self-lysis device]. <br> |
| - | The major challenge we faced when assaying for payload delivery was determining | + | The major challenge we faced when assaying for payload delivery was determining when to induce self-lysis and when to look at the choanos under the microscope. |
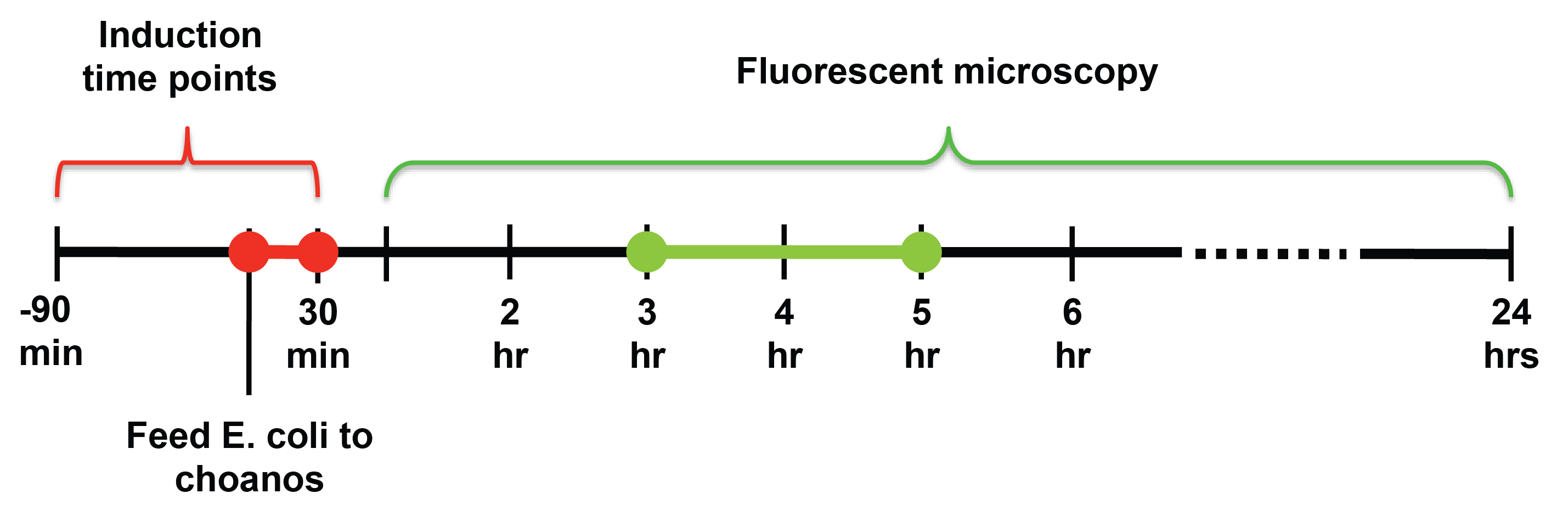
| - | For induction of self lysis, we tested timepoints from 90 minutes | + | For induction of self lysis, we tested timepoints from 90 minutes to 30 minutes after feeding the choanos (-90 to +30 minutes), with 15 minute intervals. For looking at the choanos under the microscope, we tested timepoints from 1 hour after feeding to 24 hours after feeding.<br> |
[[Image:Screen shot 2010-10-25 at 10.02.59 PM.png|700px]] | [[Image:Screen shot 2010-10-25 at 10.02.59 PM.png|700px]] | ||
| - | From our experiments, we found that induction at the same time as feeding, or 15 or 30 minutes after feeding yielded the most delivery events. For timepoints before 15 minute | + | From our experiments, we found that induction at the same time as feeding, or 15 or 30 minutes after feeding yielded the most delivery events. For timepoints before -15 minute, delivery events were undetectable or happened extremely infrequently. <br> |
We also observed that choanos seem to eat and digest bacteria with 1-2 hours after feeding, and that the best time to look for successful delivery was between 3 and 5 hours after feeding. <br> | We also observed that choanos seem to eat and digest bacteria with 1-2 hours after feeding, and that the best time to look for successful delivery was between 3 and 5 hours after feeding. <br> | ||
| - | + | ===Controls=== | |
| - | Our first step before assaying was to establish controls | + | Our first step before assaying was to establish controls. Based on how our parts should work, and work done in the Anderson Lab in mammalian cells, we expected that a sucessful delivery event would consist of diffuse GFP fluorescence throughout a choano's cytoplasm. With this in mind, the controls we ran regarding the choanos were: |
| - | + | ====Payload cells with no payload delivery device==== | |
| + | |||
| + | This control was to establish a baseline of what normal eating and digestion of bacteria in choanos looks like. We observed that choanos seem to eat with 3-5 minutes after feeding. During the first hour or so, one generally sees bacteria in food vesicles , either as rod shapes or circles (rod shaped bacteria 'head on'). They are very bright and have signifcant halos. Slowly, we end up seeing fluorescence in small circular objects.The fluorescent intensity is less than the initial bacteria in food vesicles, and we believe flourescent payload is in the food vacuole. Eventually, the fluorescence disappears altogther, degraded by the vacuole of the choano. | ||
<table> | <table> | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[Image:Choano-1hour-plain payload.jpg|thumb| | + | [[Image:Choano-1hour-plain payload.jpg|thumb|300px|Choanos fed just payload cells with no payload delivery device after 1 hour. You can see rod shaped bacteria in food vesicles in the choanos. Imaged at 100 ms, green channel exposure]] |
</td> | </td> | ||
<td> | <td> | ||
| Line 44: | Line 44: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | |||
| - | |||
| - | + | ====Payload cells with uninduced payload delivery device==== | |
| + | This control was to ascertain that any delivery events we got were from induction with arabinose, and not just a normal phenotype exhibited when choanos eat cells with self-lysis, vesicle buster and payload. | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td> | ||
| + | [[Image:Justpayload_3_(c2+c6).JPG|thumb|300px|Choanos fed just uninduced payload cells with self-lysis device and vesicle buster. We did not see any successful delivery and this image shows choanos with bacteria in their food vacuoles, in the process of normal digestion. Imaged at 300 ms, green channel exposure]] | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| - | + | ====Cells with payload and self lysis but ''no'' vesicle buster==== | |
| - | + | This control was to confirm that the successful delivery phenotype we saw was due to self-lysis device '''and''' the vesicle buster, not just self-lysis alone. We wanted to make sure that the what we were seeing was payload that had escaped the food vesicle, not just payload in a food vesicle. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td> | ||
| + | [[Image:1968-4hrs-1_(c2+c6).JPG|thumb|300px|Choanos fed just payload cells with only self-lysis after 3 hours. You can see fluorescence inside the food vacuoles of choanos. Imaged at 300 ms, green channel exposure]] | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | ====Cells with payload and vesicle buster, but ''no'' self-lysis==== | ||
| + | This control was once again to make sure that if we saw a successful delivery event, we could be certain that it was a phenotype unique to cells with induced self-lysis '''and''' vesicle buster. | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td> | ||
| + | [[Image:2347_3_(c2+c6).JPG|thumb|300px|Choanos fed payload cells with only vesicle buster. You can see bacteria in food vacuole in the choanos. Imaged at 300 ms, green channel exposure]] | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | Additionally, since all of these assays are microscopy based, we kept the settings on our microscopes constant and kept careful notes on exposure and magnification in order to avoid getting misleading results. | ||
| + | ===Payload Delivery=== | ||
| + | The following images are of successful payload delivery events. As the images of the control above showed, the phenotype of GFP throughout the cytoplasm shown below is unique to constructs with self-lysis and vesicle buster, and is consistent with what we'd expect to see based on the biology of our parts. | ||
<table> | <table> | ||
| Line 79: | Line 98: | ||
<tr> | <tr> | ||
<td> | <td> | ||
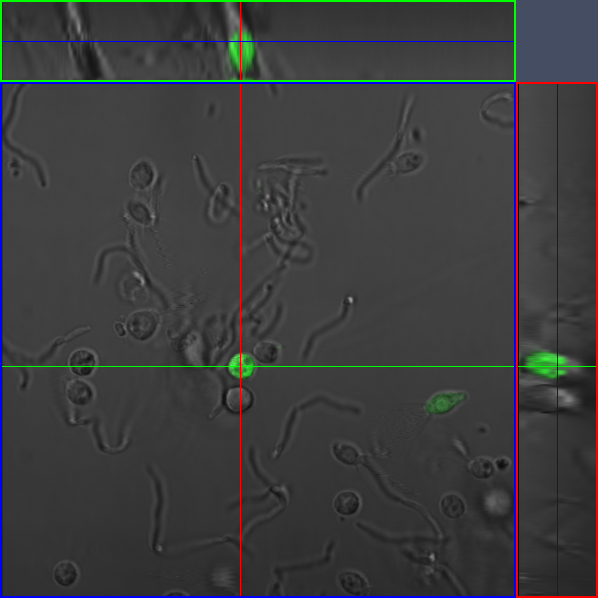
| - | + | ====Confocal Imaging==== | |
</td> | </td> | ||
<td> | <td> | ||
| Line 87: | Line 106: | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[Image:Awesome_fov2_2.jpg|thumb|300px|]] | + | [[Image:Awesome_fov2_2.jpg|thumb|300px|In this image we can see two delivery events. In the upper left corner, the choano is fixed to the surface of the viewing well in a vertical orientation, (flagella upwards) so it appears circular. In the upper right corner, the choano is lying on its side, and you can see the skirt and flagella.|]] |
</td> | </td> | ||
<td> | <td> | ||
| Line 95: | Line 114: | ||
<tr> | <tr> | ||
<td> | <td> | ||
| - | [[Image:3D_left-1.gif|thumb|300px|Z-stack imaging with 1.5 micron slices]] [[Image:3D_right.gif|thumb|300px|Z-stack imaging]] | + | [[Image:3D_left-1.gif|thumb|300px|Z-stack imaging with 1.5 micron slices.]] [[Image:3D_right.gif|thumb|300px|Z-stack imaging with 1.5 micron slices.]] |
</td> | </td> | ||
| Line 104: | Line 123: | ||
</table> | </table> | ||
| + | |||
| + | ====Efficiency of GFP Delivery: 7% (±2%)==== | ||
| + | We also used a hemocytometer to quantify the frequency of delivery. For an assay with self-lysis induction 15 minutes after feeding, an average of 7% (±2% standard deviation) of choanoflagellates experienced successful delivery of GFP - 1 out of every 16 choanos. In a culture at average density (10^6 cells per mL), we will have approximately 6.2x10^4 delivery events per mL of culture. | ||
| - | ''''' | + | '''''Protocol for Assaying''''' |
| - | + | The following are the steps we follow in order to set up and assay for payload delivery. | |
| + | # Grow up payload bacteria in TB for approximately 16 hours. | ||
| + | # We generally culture our choanos in sea water, so the first step is to aspirate off the seawater, and replace it with CGM3, a media bacteria are able to lyse in. Since choanos naturally fix to the surface of the dish they are cultured in, this step pretty straightforward. | ||
| + | # Aliquot 2 mLs of payload bacteria into two test tubes and induce one with arabinose. Put them in the 37 C shaker/incubator. This is to test for lysis before assaying using the microscope, because there is no point in assaying if the cells don't even lyse in test tubes. | ||
| + | # Induce lysis in cells. For time points that are before feeding, we induce lysis in a test tube, and put it in the shaker at 37 C until it is time to feed. For time points after feeding, we induce self-lysis and put the choanos in the 37 C incubator, without shaking. | ||
| + | # Feed the choanos. We generally feed 2 uls of bacteria per 500 ul of choanos. | ||
| + | # Put the choanos that have been fed in the 37 C incubator for 3 hours. | ||
| + | # After 3 hours, remove the choanos, and look at them under the microscope. | ||
Latest revision as of 03:10, 28 October 2010
- Home
- Project
- Parts
- Self-Lysis
- Vesicle-Buster
- Payload
- [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2010&group=Berkeley Parts Submitted]
- Results
- Judging
- Clotho
- Human Practices
- Team Resources
- Who We Are
- Notebooks:
- [http://www.openwetware.org/wiki/Berk2010-Daniela Daniela's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Christoph Christoph's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Amy Amy's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Tahoura Tahoura's Notebook]
- [http://www.openwetware.org/wiki/Berk2010-Conor Conor's Notebook]
General Experimental Set Up
Our payload delivery assay involves the following steps:
- Feed the payload bacteria to the choanos and induce self-lysis with arabinose. Induction of self lysis and feeding are not necessarily at the same time.
- Use fluorescent microscopy to detect delivery of our GFP payload.
Challenges
One of the challenges we faced in assaying for payload delivery was finding a media that the Choanoflagellates liked and that accomodated lysis. After assaying several different media formulations, including CGM, LB, TB, ASW and four different mixtures of ASW and LB, we found that CGM media was the best media for Choano viability, E. Coli lysis, and protein delivery. For more details, see our characterization of the self-lysis device.
The major challenge we faced when assaying for payload delivery was determining when to induce self-lysis and when to look at the choanos under the microscope.
For induction of self lysis, we tested timepoints from 90 minutes to 30 minutes after feeding the choanos (-90 to +30 minutes), with 15 minute intervals. For looking at the choanos under the microscope, we tested timepoints from 1 hour after feeding to 24 hours after feeding.
From our experiments, we found that induction at the same time as feeding, or 15 or 30 minutes after feeding yielded the most delivery events. For timepoints before -15 minute, delivery events were undetectable or happened extremely infrequently.
We also observed that choanos seem to eat and digest bacteria with 1-2 hours after feeding, and that the best time to look for successful delivery was between 3 and 5 hours after feeding.
Controls
Our first step before assaying was to establish controls. Based on how our parts should work, and work done in the Anderson Lab in mammalian cells, we expected that a sucessful delivery event would consist of diffuse GFP fluorescence throughout a choano's cytoplasm. With this in mind, the controls we ran regarding the choanos were:
Payload cells with no payload delivery device
This control was to establish a baseline of what normal eating and digestion of bacteria in choanos looks like. We observed that choanos seem to eat with 3-5 minutes after feeding. During the first hour or so, one generally sees bacteria in food vesicles , either as rod shapes or circles (rod shaped bacteria 'head on'). They are very bright and have signifcant halos. Slowly, we end up seeing fluorescence in small circular objects.The fluorescent intensity is less than the initial bacteria in food vesicles, and we believe flourescent payload is in the food vacuole. Eventually, the fluorescence disappears altogther, degraded by the vacuole of the choano.
Payload cells with uninduced payload delivery device
This control was to ascertain that any delivery events we got were from induction with arabinose, and not just a normal phenotype exhibited when choanos eat cells with self-lysis, vesicle buster and payload.
Cells with payload and self lysis but no vesicle buster
This control was to confirm that the successful delivery phenotype we saw was due to self-lysis device and the vesicle buster, not just self-lysis alone. We wanted to make sure that the what we were seeing was payload that had escaped the food vesicle, not just payload in a food vesicle.
Cells with payload and vesicle buster, but no self-lysis
This control was once again to make sure that if we saw a successful delivery event, we could be certain that it was a phenotype unique to cells with induced self-lysis and vesicle buster.
Additionally, since all of these assays are microscopy based, we kept the settings on our microscopes constant and kept careful notes on exposure and magnification in order to avoid getting misleading results.
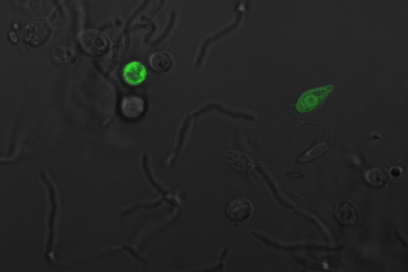
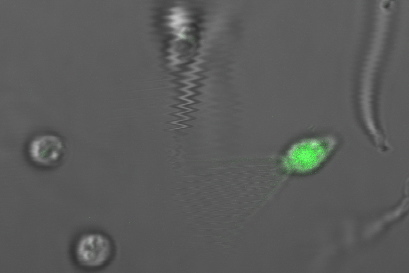
Payload Delivery
The following images are of successful payload delivery events. As the images of the control above showed, the phenotype of GFP throughout the cytoplasm shown below is unique to constructs with self-lysis and vesicle buster, and is consistent with what we'd expect to see based on the biology of our parts.
Confocal Imaging |
|
Efficiency of GFP Delivery: 7% (±2%)
We also used a hemocytometer to quantify the frequency of delivery. For an assay with self-lysis induction 15 minutes after feeding, an average of 7% (±2% standard deviation) of choanoflagellates experienced successful delivery of GFP - 1 out of every 16 choanos. In a culture at average density (10^6 cells per mL), we will have approximately 6.2x10^4 delivery events per mL of culture.
Protocol for Assaying
The following are the steps we follow in order to set up and assay for payload delivery.
- Grow up payload bacteria in TB for approximately 16 hours.
- We generally culture our choanos in sea water, so the first step is to aspirate off the seawater, and replace it with CGM3, a media bacteria are able to lyse in. Since choanos naturally fix to the surface of the dish they are cultured in, this step pretty straightforward.
- Aliquot 2 mLs of payload bacteria into two test tubes and induce one with arabinose. Put them in the 37 C shaker/incubator. This is to test for lysis before assaying using the microscope, because there is no point in assaying if the cells don't even lyse in test tubes.
- Induce lysis in cells. For time points that are before feeding, we induce lysis in a test tube, and put it in the shaker at 37 C until it is time to feed. For time points after feeding, we induce self-lysis and put the choanos in the 37 C incubator, without shaking.
- Feed the choanos. We generally feed 2 uls of bacteria per 500 ul of choanos.
- Put the choanos that have been fed in the 37 C incubator for 3 hours.
- After 3 hours, remove the choanos, and look at them under the microscope.
 "
"