Team:NYMU-Taipei/Experiments/Speedy degrader
From 2010.igem.org
(→Reporting Assay) |
(→Reporting Assay) |
||
| (13 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
=Method= | =Method= | ||
| - | |||
| - | 1.Selected genes | + | 1. Selected genes that will be reported are incubated overnight in an LB liquid culture at 37oC and 180-200rpm. This makes sure there are enough cells for experimentation by morning. Positive and negative controls are also incubated. |
| - | 2.The liquids cultured overnight are diluted into | + | 2. The liquids cultured overnight are diluted into a OD600 of 1 and incubated for 2 more hours. |
| - | 3. | + | 3. Afterwards, we took out the liquids and centrifuged them for 30 seconds at 13.2k rpm and then discarded the supernatant. We then resuspended the pellets in 2ml ABT medium. |
| - | 4. | + | 4.We measured the OD600 for the liquid, doing three replicates of 200uL, also noting the OD values. |
5.Measurement of fluorescence: | 5.Measurement of fluorescence: | ||
| - | Continuous measurement of fluorescence with the excitation/emission wavelengths depending on | + | Continuous measurement of fluorescence with the excitation/emission wavelengths depending on the fluorescent protein for one hour, with one data point per2 minutes. |
6.After measuring the fluorescence, we recorded OD value again to confirm that the E. coli has stopped growing in the ABT medium. | 6.After measuring the fluorescence, we recorded OD value again to confirm that the E. coli has stopped growing in the ABT medium. | ||
| Line 21: | Line 20: | ||
*The optimizing data: | *The optimizing data: | ||
| - | Check if the ODs remained the same to make sure the E. coli does not grow in ABT medium. We took fluorescence averages for all the replicates and sketched a curve and fitted a trend line. Exponential curves were fitted and the half-life of the FP calculated. However, the actual data | + | Check if the ODs remained the same to make sure the E. coli does not grow in ABT medium. We took fluorescence averages for all the replicates and sketched a curve and fitted a trend line. Exponential curves were fitted and the half-life of the FP calculated. However, the actual data did not fit the exponential curve as expected. |
=Reporting Assay= | =Reporting Assay= | ||
| - | '''Result''' | + | '''Result & Discussion''' |
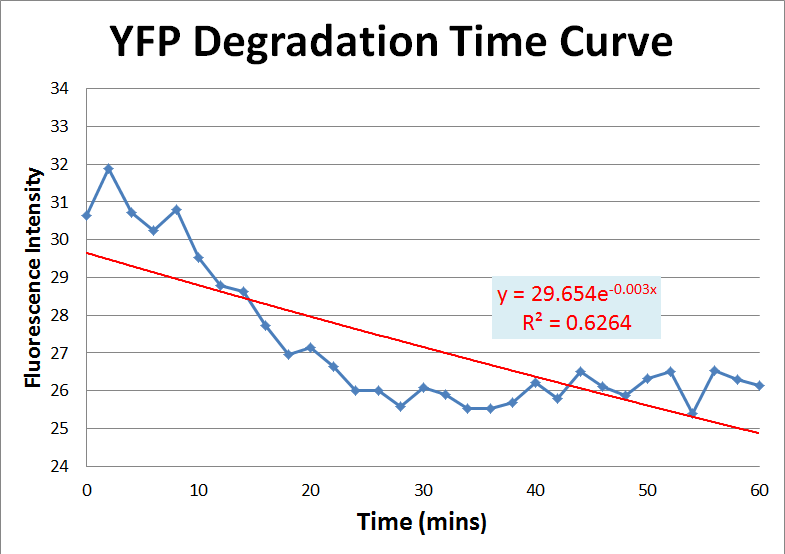
| - | *In our ssrA degradation test, we use fluorescence | + | *In our ssrA degradation test, we use fluorescence proteins to test the ssrA tag. The natural degradation half-life of the fluorescnce protein should be longer than the half life of the same protein with an added LVA tag. These graphs show that the half-life of YFP is 236 mins and 181 mins(Fig. 1 & Fig.2), while the half-time of the RFPLVA is 450 mins.(Fig. 3) |
| - | *In Fig. 4, we can see that ''E. coli'' | + | *In Fig. 4, we can see that ''E. coli'' stops growing in ABT medium as the OD600 does not vary with time. Therefore, we can infer that the amount of the ''E. coli'' remain almost constant in our fluorescence measurement. |
| - | * | + | *With the amount of ''E. coli'' constant, our data should fit into the theory. However, our curves do not fit quite well to our data. We think that it is because we do not have enough experimental data to infer an confident results. |
| - | '''Figure | + | '''Figure''' |
| - | [[image:YFP1.png|frame|none|fig.1 According to the trend line function, the half-life of the YFP is around 236 mins]] | + | [[image:YFP1.png|frame|none|fig.1 We used an exponential curve to fit the graph. The initial OD is 0.538 and the final OD is 0.507. According to the trend line function, the half-life of the YFP is around 236 mins]] |
| - | [[image:YFP2.png|frame|none| | + | [[image:YFP2.png|frame|none|fig.2 We use exponential curve to fit the graph. The initial OD is 2.484 and the final OD is 2.439. According to the trend line function, the half-life of the YFP is around 181 mins]] |
| + | |||
| + | |||
| + | |||
| + | [[image:RFPlva.png|frame|none|fig.2 We use exponential curve to fit the graph. The initial OD is 2.100 and the final OD is 2.081. According to the trend line function, the half-life of the RFPlva is around 450 mins]] | ||
{{:Team:NYMU-Taipei/Footer}} | {{:Team:NYMU-Taipei/Footer}} | ||
Latest revision as of 02:22, 28 October 2010
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Method
1. Selected genes that will be reported are incubated overnight in an LB liquid culture at 37oC and 180-200rpm. This makes sure there are enough cells for experimentation by morning. Positive and negative controls are also incubated.
2. The liquids cultured overnight are diluted into a OD600 of 1 and incubated for 2 more hours.
3. Afterwards, we took out the liquids and centrifuged them for 30 seconds at 13.2k rpm and then discarded the supernatant. We then resuspended the pellets in 2ml ABT medium.
4.We measured the OD600 for the liquid, doing three replicates of 200uL, also noting the OD values.
5.Measurement of fluorescence: Continuous measurement of fluorescence with the excitation/emission wavelengths depending on the fluorescent protein for one hour, with one data point per2 minutes.
6.After measuring the fluorescence, we recorded OD value again to confirm that the E. coli has stopped growing in the ABT medium.
- The optimizing data:
Check if the ODs remained the same to make sure the E. coli does not grow in ABT medium. We took fluorescence averages for all the replicates and sketched a curve and fitted a trend line. Exponential curves were fitted and the half-life of the FP calculated. However, the actual data did not fit the exponential curve as expected.
Reporting Assay
Result & Discussion
- In our ssrA degradation test, we use fluorescence proteins to test the ssrA tag. The natural degradation half-life of the fluorescnce protein should be longer than the half life of the same protein with an added LVA tag. These graphs show that the half-life of YFP is 236 mins and 181 mins(Fig. 1 & Fig.2), while the half-time of the RFPLVA is 450 mins.(Fig. 3)
- In Fig. 4, we can see that E. coli stops growing in ABT medium as the OD600 does not vary with time. Therefore, we can infer that the amount of the E. coli remain almost constant in our fluorescence measurement.
- With the amount of E. coli constant, our data should fit into the theory. However, our curves do not fit quite well to our data. We think that it is because we do not have enough experimental data to infer an confident results.
Figure
 "
"