Team:Stockholm/27 September 2010
From 2010.igem.org
(→Andreas) |
m |
||
| (6 intermediate revisions not shown) | |||
| Line 26: | Line 26: | ||
====Gel verification==== | ====Gel verification==== | ||
| + | |||
'''Gel 1'''<br /> | '''Gel 1'''<br /> | ||
1 % agarose, 110 V | 1 % agarose, 110 V | ||
| Line 45: | Line 46: | ||
'''Results'''<br /> | '''Results'''<br /> | ||
| - | Gels run too | + | Gels run too far (no data). New gels will be run tomorrow. |
===Sequencing=== | ===Sequencing=== | ||
Samples prepared 25/9 were sent for sequencing. Sequencing information added to the 25/9 notebook page. | Samples prepared 25/9 were sent for sequencing. Sequencing information added to the 25/9 notebook page. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Mimmi == | ||
| + | |||
| + | === Over expression === | ||
| + | |||
| + | {| | ||
| + | | Start cultures | ||
| + | |- | ||
| + | | *3ml LB<sub>AMP</sub> + tip from glycerol stock | ||
| + | |- | ||
| + | | *Grow ON in 37°C, 225rpm | ||
| + | |- | ||
| + | | '''DNA''' | ||
| + | |- | ||
| + | | pEX.SOD | ||
| + | |- | ||
| + | | pEX.yCCS | ||
| + | |- | ||
| + | | pEX.SOD.his | ||
| + | |- | ||
| + | | pEX.his.SOD | ||
| + | |} | ||
| + | |||
| + | ==Nina== | ||
| + | |||
| + | ===Sequencing=== | ||
| + | |||
| + | I send two samples for sequencing. 15 ul sample and 1.5 ul Forward primer. | ||
| + | |||
| + | *pMa #3 Tyrosinase_N ASB0045 694 | ||
| + | *Tyrosinase in bank vector K ASB0045 695 | ||
| + | |||
| + | ===Digestion=== | ||
| + | |||
| + | Digestion of Fusion (1/9): | ||
| + | |||
| + | *H2O 15 ul | ||
| + | *DNA 2 ul | ||
| + | *Fastdigest buffer 10X 2 ul | ||
| + | *Restriction enzyme NgoMIV 1 ul | ||
| + | *Restriction enzyme SpeI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min) | ||
| + | |||
| + | Digestion of Fusion (1/9): | ||
| + | |||
| + | *H2O 15 ul | ||
| + | *DNA 2 ul | ||
| + | *Fastdigest buffer 10X 2 ul | ||
| + | *Restriction enzyme AgeI 1 ul | ||
| + | *Restriction enzyme EcoRI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min) | ||
| + | |||
| + | Inactivate 20 min 80 °C all samples | ||
| + | |||
| + | Digestion of IgG #5_E_pMa: | ||
| + | |||
| + | *H2O 15 ul | ||
| + | *DNA 2 ul | ||
| + | *Fastdigest buffer 10X 2 ul | ||
| + | *Restriction enzyme NgoMIV 1 ul | ||
| + | *Restriction enzyme PstI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min) | ||
| + | |||
| + | |||
| + | Digestion of IgG #5_E_pMa: | ||
| + | |||
| + | *H2O 15 ul | ||
| + | *DNA 2 ul | ||
| + | *Fastdigest buffer 10X 2 ul | ||
| + | *Restriction enzyme AgeI 1 ul | ||
| + | *Restriction enzyme EcoRI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min) | ||
| + | |||
| + | |||
| + | Digestion of Protein A#5_E_pMa: | ||
| + | |||
| + | *H2O 15 ul | ||
| + | *DNA 2 ul | ||
| + | *Fastdigest buffer 10X 2 ul | ||
| + | *Restriction enzyme AgeI 1 ul | ||
| + | *Restriction enzyme EcoRI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min) | ||
| + | |||
| + | Digestion of Protein A#5_CPPs_N: | ||
| + | |||
| + | *H2O 15 ul | ||
| + | *DNA 2 ul | ||
| + | *Fastdigest buffer 10X 2 ul | ||
| + | *Restriction enzyme XbaI 1 ul | ||
| + | *Restriction enzyme PstI 1 ul | ||
| + | |||
| + | Incubate in 37 °C for 30 min. | ||
| + | |||
| + | ===Concentration measurement=== | ||
| + | |||
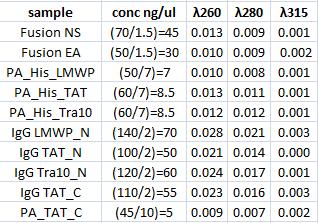
| + | I measured conc of the samples for preparation of the following ligation. | ||
| + | |||
| + | [[Image:Aq31.jpg|300px]] | ||
| + | |||
| + | ===Ligation=== | ||
| + | |||
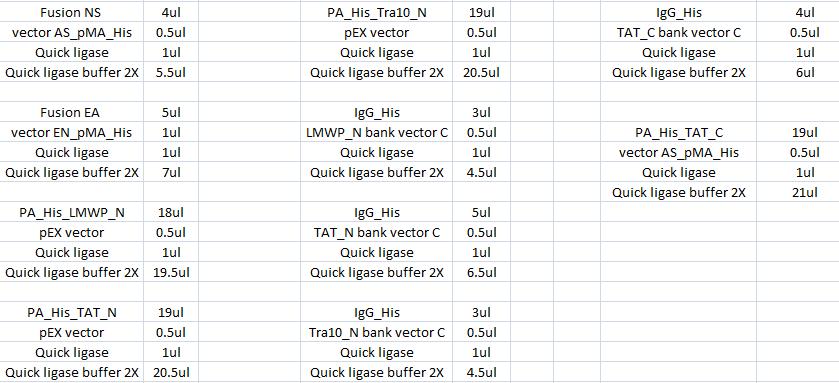
| + | The ligation was according to: | ||
| + | |||
| + | [[Image:Aq32.jpg|700px]] | ||
| + | |||
| + | I incubated the ligations in a water bath, 22 °C in 15 min. | ||
| + | |||
| + | ==Johan== | ||
| + | |||
| + | ===MIniprep=== | ||
| + | Two his-bFGF and two bFGF-his samples. | ||
| + | |||
| + | All ~400 ng/µl | ||
| + | |||
| + | ===Digestion=== | ||
| + | |||
| + | 2 µl DNA | ||
| + | 2 µl 10x fastbuffer | ||
| + | (1 µl BamHI) | ||
| + | 15 µl H2O | ||
| + | |||
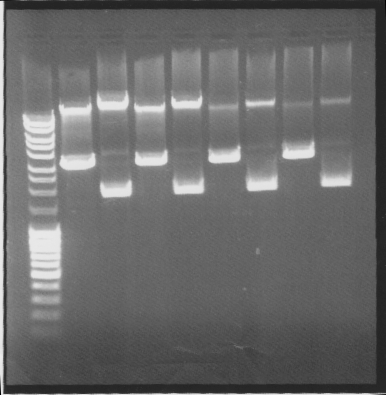
| + | ===Gel=== | ||
| + | |||
| + | Cut & uncut | ||
| + | |||
| + | [[Image:SU 27sepgel.png]] | ||
| + | |||
| + | Results: All showed good results | ||
| + | |||
| + | ===Cut bFGF-his=== | ||
| + | |||
| + | 3 µl DNA | ||
| + | |||
| + | 1 µl NgoMIV | ||
| + | |||
| + | 1 µl PstI | ||
| + | |||
| + | 2 µl 10x fastbuffer | ||
| + | |||
| + | 13 µl H2O | ||
| + | |||
| + | Then heat-inactivation | ||
| + | |||
| + | ===Ligation=== | ||
| + | |||
| + | Of cut bFGF-his into already cut TAT_N vector | ||
| + | |||
| + | 5 µl bFGF | ||
| + | |||
| + | 1 µl vector | ||
| + | |||
| + | 2 µl 10x fastbuffer | ||
| + | |||
| + | 1 µl T4 ligase | ||
| + | |||
| + | 11 µl H2O | ||
| + | |||
| + | 1h 37 °C | ||
| + | |||
| + | ===Transformation | ||
| + | |||
| + | 3 µl of all constructs was transformed into top10 cells | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Latest revision as of 21:53, 27 October 2010
Contents |
Andreas
Cloning and assembly
Transformation results
From 25/9 transformations
Good colony yield on all plates. Good white:red colony ratio on pEX plates (Constructs 1, 2, 3 and 4).
Colony PCR
Picked colonies for colony PCR.
- pEX.N-LMWP⋅SOD⋅His (K): 1-2
- pEX.N-TAT⋅SOD⋅His: 1-2
- pEX.N-Tra10⋅SOD⋅His: 1-2
- pEX.N-LMWP⋅SOD⋅His (C): 1-2
- pSB1K3.N-LMWP⋅SOD⋅His.RBS.yCCS: 1-4
- pSB1K3.N-TAT⋅SOD⋅His.RBS.yCCS: 1-4
- pSB1K3.N-Tra10⋅SOD⋅His.RBS.yCCS: 1-4
- pSB1C3.N-LMWP⋅SOD⋅His.RBS.yCCS: 1-4
- BL21 pEX.N-TAT⋅SOD⋅His 3: 1-2
- BL21 pEX.N-TAT⋅SOD⋅His 4: 1-2
Standard colony PCR protocol.
- Elongation time: 2:00
Gel verification
Gel 1
1 % agarose, 110 V
Gel 2
0.8 % agarose, 90 V
Expected bands
- 744 bp
- 735 bp
- 765 bp
- 744 bp
- 1645 bp
- 1636 bp
- 1666 bp
- 1645 bp
- 735 bp
- 735 bp
Results
Gels run too far (no data). New gels will be run tomorrow.
Sequencing
Samples prepared 25/9 were sent for sequencing. Sequencing information added to the 25/9 notebook page.
Mimmi
Over expression
| Start cultures |
| *3ml LBAMP + tip from glycerol stock |
| *Grow ON in 37°C, 225rpm |
| DNA |
| pEX.SOD |
| pEX.yCCS |
| pEX.SOD.his |
| pEX.his.SOD |
Nina
Sequencing
I send two samples for sequencing. 15 ul sample and 1.5 ul Forward primer.
- pMa #3 Tyrosinase_N ASB0045 694
- Tyrosinase in bank vector K ASB0045 695
Digestion
Digestion of Fusion (1/9):
- H2O 15 ul
- DNA 2 ul
- Fastdigest buffer 10X 2 ul
- Restriction enzyme NgoMIV 1 ul
- Restriction enzyme SpeI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min)
Digestion of Fusion (1/9):
- H2O 15 ul
- DNA 2 ul
- Fastdigest buffer 10X 2 ul
- Restriction enzyme AgeI 1 ul
- Restriction enzyme EcoRI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min)
Inactivate 20 min 80 °C all samples
Digestion of IgG #5_E_pMa:
- H2O 15 ul
- DNA 2 ul
- Fastdigest buffer 10X 2 ul
- Restriction enzyme NgoMIV 1 ul
- Restriction enzyme PstI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min)
Digestion of IgG #5_E_pMa:
- H2O 15 ul
- DNA 2 ul
- Fastdigest buffer 10X 2 ul
- Restriction enzyme AgeI 1 ul
- Restriction enzyme EcoRI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min)
Digestion of Protein A#5_E_pMa:
- H2O 15 ul
- DNA 2 ul
- Fastdigest buffer 10X 2 ul
- Restriction enzyme AgeI 1 ul
- Restriction enzyme EcoRI 1 ul (Add after 1.5 h in 37 °C & incubate in additional 30 min)
Digestion of Protein A#5_CPPs_N:
- H2O 15 ul
- DNA 2 ul
- Fastdigest buffer 10X 2 ul
- Restriction enzyme XbaI 1 ul
- Restriction enzyme PstI 1 ul
Incubate in 37 °C for 30 min.
Concentration measurement
I measured conc of the samples for preparation of the following ligation.
Ligation
The ligation was according to:
I incubated the ligations in a water bath, 22 °C in 15 min.
Johan
MIniprep
Two his-bFGF and two bFGF-his samples.
All ~400 ng/µl
Digestion
2 µl DNA 2 µl 10x fastbuffer (1 µl BamHI) 15 µl H2O
Gel
Cut & uncut
Results: All showed good results
Cut bFGF-his
3 µl DNA
1 µl NgoMIV
1 µl PstI
2 µl 10x fastbuffer
13 µl H2O
Then heat-inactivation
Ligation
Of cut bFGF-his into already cut TAT_N vector
5 µl bFGF
1 µl vector
2 µl 10x fastbuffer
1 µl T4 ligase
11 µl H2O
1h 37 °C
===Transformation
3 µl of all constructs was transformed into top10 cells
 |

|
 |

|
 |

|

|

|
 "
"