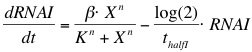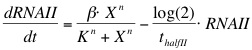Team:Groningen/Killswitch model
From 2010.igem.org
(→Kill Switch) |
(→Kill Switch) |
| (2 intermediate revisions not shown) | |
Latest revision as of 19:25, 27 October 2010
Kill Switch
Toxin/antidote system
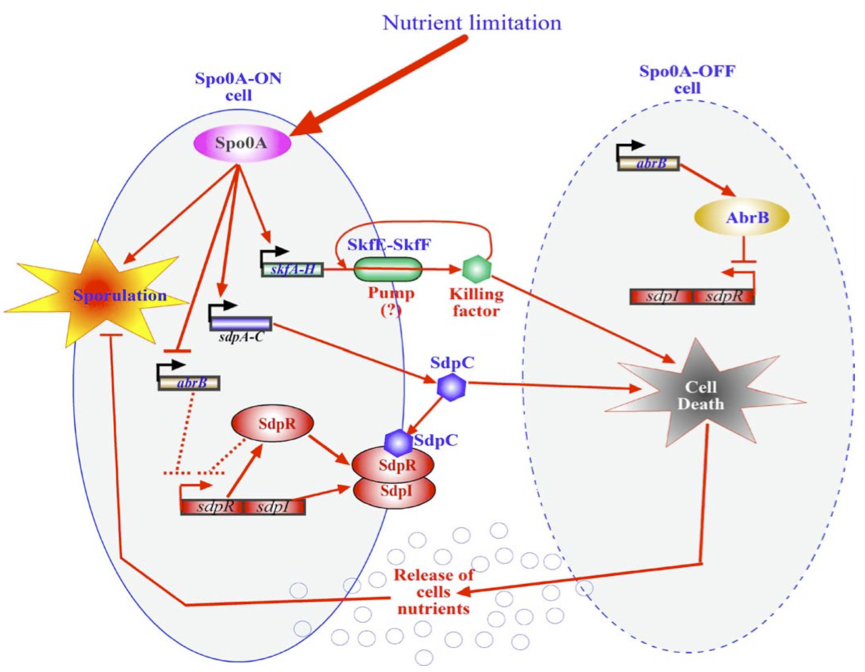
Killswitch coupled to sporulation in Bacillus subtilis
Another way of killing the cells once the biofilm has formed would be to alter an already existing in B. subtillis Within Bacillus subtilis a system exists whereby the sporulation process is delayed by killing surrounding cells. Cells that are Spo0A-Off are lysed whereby nutrients are released. These nutrients can be consumed by the Spo0A-On cells thereby delaying the process of sporulation The skf and sdp operons are responsible for the death of a subpopulation of sporulating bacterial cells. The regulatory protein Spo0A up regulates the sporulating killing factor (skfA-H operon) and the sporulating delay protein (sdpABC operon). Surrounding cells that are Spo0A-Off are killed by the extracellular killing factor skf, produced by Spo0A-On cells. SkfE and SkfF together probably act as an export pump that secretes the killing factor and thereby protect the cell. Another killing factor is sdpC. The Spo0A-On cells have two mechanisms that provide resistance to sdpC. The first one is the SdpC-SdpI-SdpR signaling pathway. The second is the repression of AbrB synthesis. A schematic overview of this mechanism is given below. If any of the factors that protect the cells from their own toxins were dysfunctional, the cells would kill themselves as soon as the Spo0A system would be turned on due nutrient limitation (which will happen inevitably in a biofilm after a while).
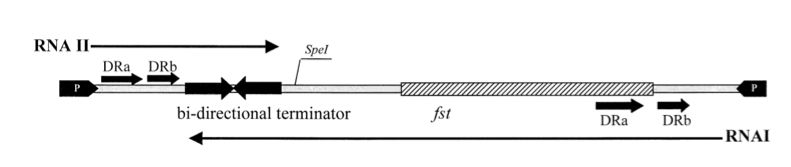
Anti-sense RNA regulation
In Gram-positive bacteria antisense RNA-regulated stability is found.
The so-called par region encodes for two small RNAs , RNAI and RNAII as depicted in the above figure. RNAI is 210nt long and contains the fst gene which encodes the toxic compound. RNAII is only 65nt long and highly complementary to RNAI. This results in complex formation whereby RNAI is not able to translate the fst gene into the faecalis plasmid-stabilizing toxin compound. The half-life time of RNAI is only 10 minutes while the half-life time of RNAII is more than one hour. (Zielenkiewicz)(Greenfield) This difference can be used as some sort of timer on a killswitch since the toxin concentration will eventually outcompete the antitoxin concentration. Our model is based on this kill switch system.
Promoters
To ensure a bigger time delay between chaplin expression and kill switch activation, the srfA promoter could start the chaplin expression, whereas the yqxM promoter which is active later in the growth curve would start the kill switch.
The activation of the par region can be modeled with a simple activator term. The degradation of the two RNAs can be modelled with half life equations. The change in biomass can be modeled with a simple growth model combined with
the delta tox.
The model
The formation of RNAI and RNAII is described with the Hill's equation. The degradation of the RNA is taken into account by using the half-life of the different RNA's. The Hill's equation is the same for both RNA's because the assumption is made that both promoters are equal in strength.
In the following table all constants used in the model are given.
The assumption is made that the promoter is very strong, therefore the maximal expression level of the promoter is assumed to be 1 mRNA molecule per second. Normally promoter expression levels range between 10-4 and 1 mRNA molecules per second. K is the activation coefficient and defines the concentration active X needed to significantly activate expression. The concentration of transcription factor is assumed to be much higher than the activation coefficient K. A value of 500 nM is chosen for this model.
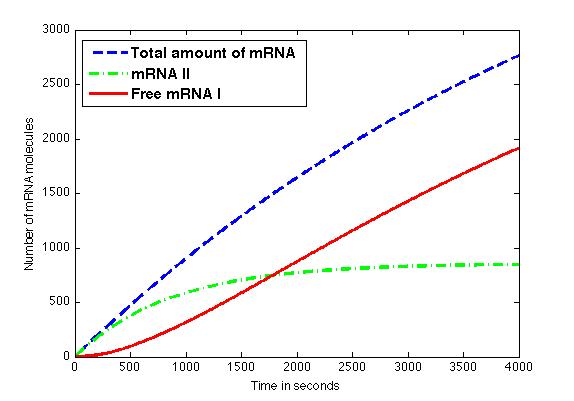
The figure shows the amount of mRNA molecules in time. Assuming that all available mRNA II binds to mRNA I, the red line in the graph shows the amount of free mRNA I molecules. Translation of these mRNA I molecules results in the formation of a toxic compound. Above a certain threshold this will result in cell death.
Matlab source files
Killswitch ([http://dl.dropbox.com/u/391356/iGEM/killswitchMatlab.zip source])
References
Gazit E and Sauer RT, The Doc Toxin and Phd antidote proteins of the bacteriophage P1 plasmid addiction system form a heterotrimeric complex. Journal of Biological Chemistry, 1999, 274 (24):16813-8. PMID 10358024
Greenfield TJ, Ehli E, Kirshenmann T, Franch T, Gerdes K, Weaver KE, The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism, Molecular Microbiology, 2000, 37(3): 652-660. PMID 10931358
Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R, Bacterial programmed cell death and multicellular behavior in bacteria, Plos genetics 2(10): e135. PMID 15036322
Zielenkiewicz U and Ceglowski P, Mechanisms of plasmid stable maintenance with special focus on plasmid addiction ystems, Acta Biochimica Polonia, 2001, 48 (4): 1003-1023. PMID 11995964
 "
"