Team:EPF Lausanne/Project droso
From 2010.igem.org
(→Experiments on Drosophilia) |
|||
| (79 intermediate revisions not shown) | |||
| Line 18: | Line 18: | ||
<div CLASS="EPFL_content"> | <div CLASS="EPFL_content"> | ||
| - | + | =Introduction= | |
| - | |||
| - | + | [[Image:Mousquito and asaia.png|right|200px|caption]] | |
| - | Considering the fact that bacteria that live in the guts of insects are not very common, we assumed that there was a fair chance that Asaia could persist in Drosophila and that we could use | + | The final goal of our project is for our modified Asaia to survive and produce proteins in the mosquito's gut. However, working with mosquitoes requires special equipment that we do not have at EPFL. We wondered if we could work on another insect which is less demanding, and therefore turned towards ''Drosophila'' (commonly known as the fruit fly), which is much easier to work with. |
| + | |||
| + | Considering the fact that bacteria that live in the guts of insects are not very common, we assumed that there was a fair chance that Asaia could persist in ''Drosophila'' and that we could use it as an alternative to mosquitoes for our basic experiments. | ||
| + | |||
| + | We therefore decided to initially work with ''Drosophila'', which we discuss in the first part of this section, and later turn to mosquitoes, which we discuss in the second part of this section. | ||
| + | |||
| + | = I) Experiments on ''Drosophila'' = | ||
| - | + | With ''Drosophila melanogaster'' we aimed to address two questions: | |
| - | i | + | <br> i. Is ''Asaia'' toxic for ''Drosophila''? |
| - | ii | + | <br> ii.Is ''Asaia'' able to colonize the Drosophila'' gut and persist? |
| - | = | + | (See [https://2010.igem.org/wiki/index.php?title=Team:EPF_Lausanne/Project/Materials_Methods_Drosophila Materials and Methods] for details on how the experiments were conducted.) |
| - | == | + | == Our main results == |
| - | + | [[Image:Asaiaflies.png|center|600px|thumb|bottom|'''Figure 1''' Observing ''Asaia'' that express GFP in vivo]] | |
| - | |||
| - | |||
| - | + | ===1) ''Asaia'' is not toxic for Drosophila=== | |
| + | [[Image:Survival.png|center|600px|thumb|bottom|'''Figure 2''' ''Asaia'' is not toxic to ''Drosophila''. Immunodeficient ''Relish'' (A) and wild-type ''Oregon'' (B) flies were infected with different bacterial strains. A lethal control strain (''P. entomophila''), a non-lethal control strain (''Ecc 15'') and our ''Asaia'' bacteria. Dead flies were counted over the course of the experiment..]] | ||
| - | = | + | We infected ''Drosophila'' with different [https://2010.igem.org/wiki/index.php?title=Team:EPF_Lausanne/Project/Materials_Methods_Drosophila bacterial strains], a pathogenic control starin (''P. entomophila''), a non-pathogenic control strain (Ecc 15) and our ''Asaia'' bacteria. For these experiments we used two different [https://2010.igem.org/wiki/index.php?title=Team:EPF_Lausanne/Project/Materials_Methods_Drosophila fly strains] (''Oregon'' and ''Relish''). |
| - | + | ||
| - | We | + | We found that ''Asaia'' does not cause significantly more deaths than the non-pathogenic bacteria or the uninfected control (Figure 2). |
| - | + | ===2) ''Asaia'' is not persistent in ''Drosophila'' === | |
| - | + | [[Image:Persistance.png|center|400px|thumb|bottom|'''Figure 3''' ''Asaia'' does not persist in ''Drosophila''.]] | |
| - | + | With this last experiment we wanted to ascertain wether ''Asaia'' persisted in ''Drosophila'' and monitor how many ''Asaia'' were present in the ''Drosophila’s'' gut after 3h, 24h and 48h. | |
| - | + | To be able to “count” the number of ''Asaia'' at these three different periods of time, we had to retrieve the bacteria inside the flies, plate them and after incubation count the number of colonies present. | |
| - | '' | + | To do this the flies were crushed in the medium corresponding to the bacteria we were interested in (i.e: Gly for ''Asaia'', LB for ''Pe'', etc). We then did a serial dilution eleven times with a factor of ten with the crushed flies, and plated each dilution with antibiotics to specifically select the bacteria we were interested in. |
| - | + | ==Conclusion== | |
| - | + | The survival assay reveals that "Asaia" is not lethal for "Drosophila". | |
| - | + | The persistence assay showed that "Asaia" is unable to establish itself in the fruit fly's gut. This prevents us to use "Drosophila" as a model for Asaia insect interactions. | |
| - | + | As mentioned before, bacteria that live in insects’ gut are not common and it is possible that because it cannot survive in "Drosophila", it is actually very specific to mosquitoes. With this assumption, we could assume that we can introduce our modified "Asaia" into the wildlife with very little risk of it spreading to other insects. | |
| + | = II) Experiments on mosquitoes = | ||
| + | The final objective of this project is to determine if mosquitoes carrying our engineered Asaia are less capable of transmitting malaria. However, we do not have mosquito swarms here in EPFL. To do this experiment, we contacted the Institute Pasteur in Paris to collaborate with us and proceed with our experiments. Nevertheless, the experiments will not be done before the iGEM jamboree... | ||
{{EPFL_2010_bottom_wrap}} | {{EPFL_2010_bottom_wrap}} | ||
</div> | </div> | ||
Latest revision as of 19:09, 27 October 2010


Contents |
Introduction
The final goal of our project is for our modified Asaia to survive and produce proteins in the mosquito's gut. However, working with mosquitoes requires special equipment that we do not have at EPFL. We wondered if we could work on another insect which is less demanding, and therefore turned towards Drosophila (commonly known as the fruit fly), which is much easier to work with.
Considering the fact that bacteria that live in the guts of insects are not very common, we assumed that there was a fair chance that Asaia could persist in Drosophila and that we could use it as an alternative to mosquitoes for our basic experiments.
We therefore decided to initially work with Drosophila, which we discuss in the first part of this section, and later turn to mosquitoes, which we discuss in the second part of this section.
I) Experiments on Drosophila
With Drosophila melanogaster we aimed to address two questions:
i. Is Asaia toxic for Drosophila?
ii.Is Asaia able to colonize the Drosophila gut and persist?
(See Materials and Methods for details on how the experiments were conducted.)
Our main results
1) Asaia is not toxic for Drosophila
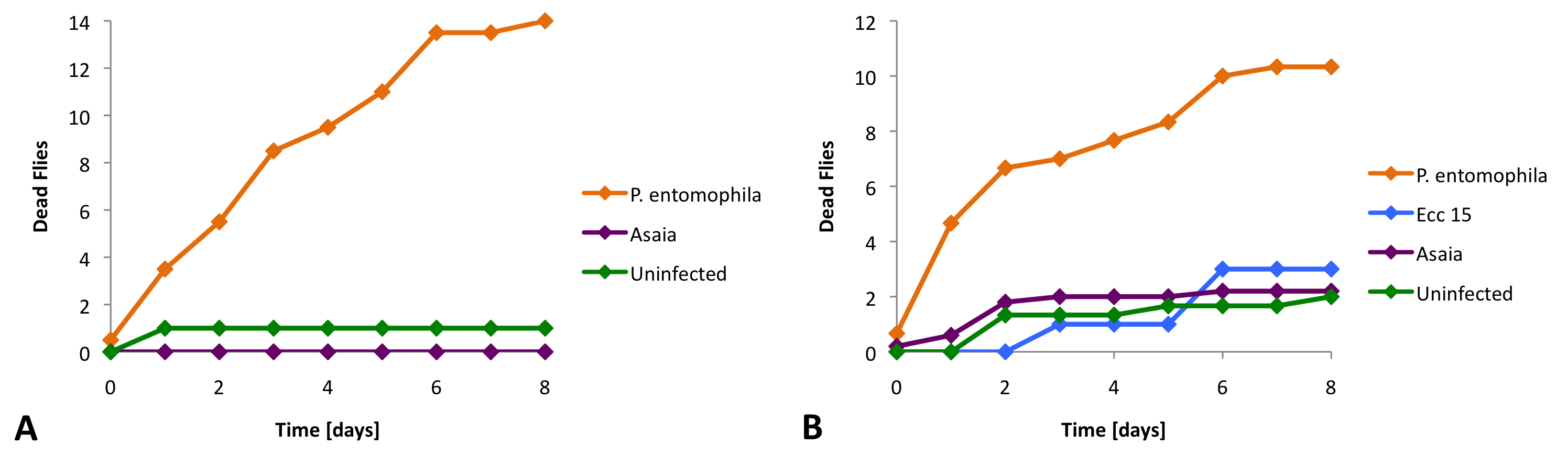
We infected Drosophila with different bacterial strains, a pathogenic control starin (P. entomophila), a non-pathogenic control strain (Ecc 15) and our Asaia bacteria. For these experiments we used two different fly strains (Oregon and Relish).
We found that Asaia does not cause significantly more deaths than the non-pathogenic bacteria or the uninfected control (Figure 2).
2) Asaia is not persistent in Drosophila
With this last experiment we wanted to ascertain wether Asaia persisted in Drosophila and monitor how many Asaia were present in the Drosophila’s gut after 3h, 24h and 48h.
To be able to “count” the number of Asaia at these three different periods of time, we had to retrieve the bacteria inside the flies, plate them and after incubation count the number of colonies present.
To do this the flies were crushed in the medium corresponding to the bacteria we were interested in (i.e: Gly for Asaia, LB for Pe, etc). We then did a serial dilution eleven times with a factor of ten with the crushed flies, and plated each dilution with antibiotics to specifically select the bacteria we were interested in.
Conclusion
The survival assay reveals that "Asaia" is not lethal for "Drosophila".
The persistence assay showed that "Asaia" is unable to establish itself in the fruit fly's gut. This prevents us to use "Drosophila" as a model for Asaia insect interactions.
As mentioned before, bacteria that live in insects’ gut are not common and it is possible that because it cannot survive in "Drosophila", it is actually very specific to mosquitoes. With this assumption, we could assume that we can introduce our modified "Asaia" into the wildlife with very little risk of it spreading to other insects.
II) Experiments on mosquitoes
The final objective of this project is to determine if mosquitoes carrying our engineered Asaia are less capable of transmitting malaria. However, we do not have mosquito swarms here in EPFL. To do this experiment, we contacted the Institute Pasteur in Paris to collaborate with us and proceed with our experiments. Nevertheless, the experiments will not be done before the iGEM jamboree...

 "
"