Team:UNAM-Genomics Mexico/Modules/In vivo
From 2010.igem.org
Kurupaclau (Talk | contribs) |
Kurupaclau (Talk | contribs) |
||
| Line 48: | Line 48: | ||
=====OBJECTIVES===== | =====OBJECTIVES===== | ||
| - | The final aim was the construction of the blue light emission bacterial luciferase (LuxAB)from Vibrio fischeri. | + | *The final aim was the construction of the blue light emission bacterial luciferase (LuxAB)from Vibrio fischeri. |
The structure of the device was designed as follows: | The structure of the device was designed as follows: | ||
| Line 55: | Line 55: | ||
Because the luciferase coded by luxA and luxB needs a substrate (an aldehyde synthesized by enzymes into the lux operon) and we were not planning the design of a biobrick containing the genes needed for its synthesis we will add the aldehyde to the reaction in an exogenous way. | Because the luciferase coded by luxA and luxB needs a substrate (an aldehyde synthesized by enzymes into the lux operon) and we were not planning the design of a biobrick containing the genes needed for its synthesis we will add the aldehyde to the reaction in an exogenous way. | ||
| + | |||
| + | *Design LuxY biobrick, send to synthesise and transfer the construction from Mr. Gene plasmid to pSB1C3 DNA submission backbone. | ||
| + | |||
| + | *To test that the device works as expected. | ||
| Line 104: | Line 108: | ||
Double Terminator: BBa_B0015 | Double Terminator: BBa_B0015 | ||
| - | See [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360100 LuxY | + | See [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360100 LuxY -Part:BBa_K360100-] annotation at the registry for more details. |
| Line 119: | Line 123: | ||
=====METHODOLOGY===== | =====METHODOLOGY===== | ||
| + | |||
====Emission module: LuxAB luciferase from vibrio fischeri plus Lumazine protein==== | ====Emission module: LuxAB luciferase from vibrio fischeri plus Lumazine protein==== | ||
| + | |||
---- | ---- | ||
=====OBJECTIVES===== | =====OBJECTIVES===== | ||
| + | *Obtain LuxAB genes from Vibrio fischeri. | ||
| + | |||
| + | *Design Lumazine biobrick, send to synthesise and transfer the construction from Mr. Gene plasmid to pSB1C3 DNA submission backbone. | ||
| + | |||
| + | *To test that the device works as expected. This characterisation will test if Lumazine protein can also be functional working with the Vibrio fischeri luciferase, instead of its native interacting luciferase from Photobacterium phosphoreum. | ||
=====METHODOLOGY===== | =====METHODOLOGY===== | ||
| + | |||
| + | =====Lux operon===== | ||
| + | |||
| + | The strategy to obtain LuxAB genes from Vibrio fischeri was the aforementioned working with the green light emission. | ||
| + | |||
| + | =====Lumazine===== | ||
| + | |||
| + | Lumazine protein of Photobacterium phosphoreum (lumP) is a small protein which shifts the wavelength of light emitted by the P.phosphoreum luciferase LuxAB from 495 nm to about 475 nm, we needed it to tune the blue light emission wavelength towards another one closer to that of LovTap system reception. | ||
| + | |||
| + | '''A version of the lumazine protein was sent to us by Edinburgh Team''', due to this construction hadn't been tested we decided to synthesise it, in order to avoid unexpected results for possible errors in the already assembled biobrick from Edinburgh. | ||
| + | |||
| + | This is the composition of the synthesized sequence, “Gene” stands only for lumazine coding region. | ||
| + | |||
| + | Preffix+ Promoter + RBS + Gene + Double Terminator + Suffix | ||
| + | |||
| + | Promoter: BBa_J23119 | ||
| + | |||
| + | RBS: BBa_B0034 | ||
| + | |||
| + | Gene: Vibrio fischeri yellow fluorescent protein (lumazine) | ||
| + | |||
| + | Double Terminator: BBa_B0015 | ||
| + | |||
| + | See [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360122 Lumazine -Part:BBa_K360122-] annotation at the registry for more details. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 146: | Line 185: | ||
*Construct an improved version of the LovTAP photosensor device under the regulation of different constitutive promoters, due to the previous version of LovTAP designed by 2009 Lausanne team didn´t have a differential behavior under the inverting regulator sensitive to LacI and CAP protein, their results showed that the expression levels of LovTAP didn’t show differences to the induction with IPTG. As well, we included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne. With this mutation LovTAP should react faster and the conformational change should be more stable (the protein stays in the active form for longer, under light induction). | *Construct an improved version of the LovTAP photosensor device under the regulation of different constitutive promoters, due to the previous version of LovTAP designed by 2009 Lausanne team didn´t have a differential behavior under the inverting regulator sensitive to LacI and CAP protein, their results showed that the expression levels of LovTAP didn’t show differences to the induction with IPTG. As well, we included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne. With this mutation LovTAP should react faster and the conformational change should be more stable (the protein stays in the active form for longer, under light induction). | ||
| + | |||
| + | *Transfer the plasmid with the LovTAP synthesised gene from Mr. Gene Plasmid to pSB1C3 DNA submission backbone and ligate the construction to different weak constitutive promoters. | ||
*Characterize LovTAP reporter systems, both repressor and activator activity. | *Characterize LovTAP reporter systems, both repressor and activator activity. | ||
| Line 171: | Line 212: | ||
5. The stop codon tga was changed for two taa. | 5. The stop codon tga was changed for two taa. | ||
| - | For more details visit our team [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360121 LovTAP | + | For more details visit our team [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360121 LovTAP -Part:BBa_K360121-] entry at the registry page. |
| Line 277: | Line 318: | ||
[[Image:Red luciferase.jpg]] | [[Image:Red luciferase.jpg]] | ||
| + | |||
| + | *Design LRE biobrick and transfer the plasmid with the synthesised gene from Mr. Gene Plasmid to pSB1C3 DNA submission backbone and assemble it to a constitutive promoter. | ||
*To test that the device works as expected. | *To test that the device works as expected. | ||
| Line 305: | Line 348: | ||
Our goal here was to couple both, luciferase and LRE, to be able to make the construction autonomous after the first and single input of luciferin. We sent to synthesize the LRE enzyme. | Our goal here was to couple both, luciferase and LRE, to be able to make the construction autonomous after the first and single input of luciferin. We sent to synthesize the LRE enzyme. | ||
| - | For more details see [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360113 LRE | + | For more details see [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360113 LRE -Part:BBa_K360113-] entry at the Registry page. |
}} | }} | ||
Revision as of 14:04, 27 October 2010

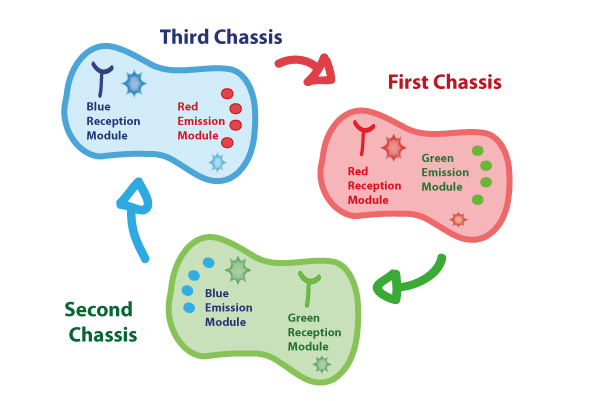
Coupling together Biological Chassis
In order to enable the light based communication between bacteria, we have designed a tertiary cycle of different bacteria chassis, assembling each module of reception and emission to construct a light-based feedback loop of red-green-blue light, which will make the proof of concept of communication over distance and proper signal decoding.
Tertiary cycle
First Chassis
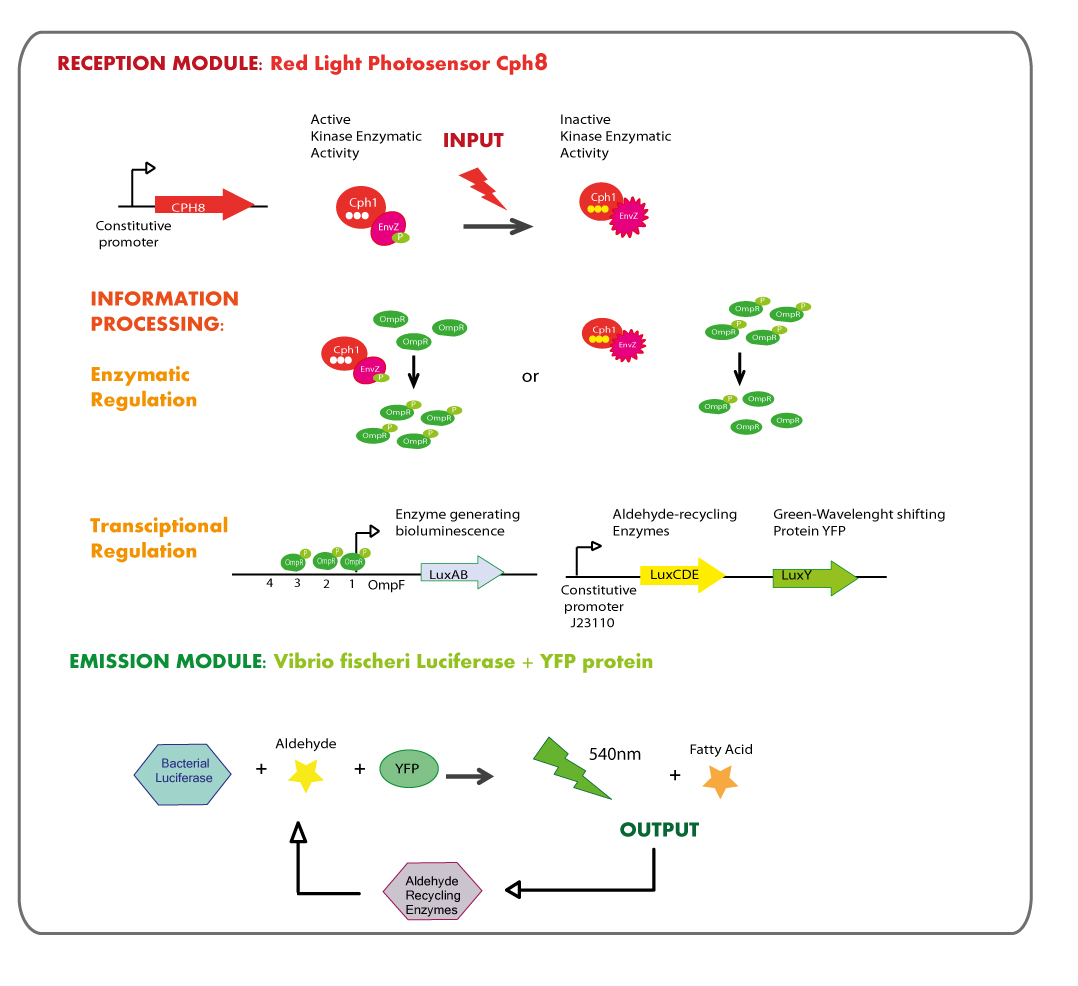
Reception module: Red Light Photosensor cph8
OBJECTIVES
METHODOLOGY
Emission module: LuxAB luciferase from vibrio fischeri plus YFP protein
OBJECTIVES
- The final aim was the construction of the blue light emission bacterial luciferase (LuxAB)from Vibrio fischeri.
The structure of the device was designed as follows:
Because the luciferase coded by luxA and luxB needs a substrate (an aldehyde synthesized by enzymes into the lux operon) and we were not planning the design of a biobrick containing the genes needed for its synthesis we will add the aldehyde to the reaction in an exogenous way.
- Design LuxY biobrick, send to synthesise and transfer the construction from Mr. Gene plasmid to pSB1C3 DNA submission backbone.
- To test that the device works as expected.
METHODOLOGY
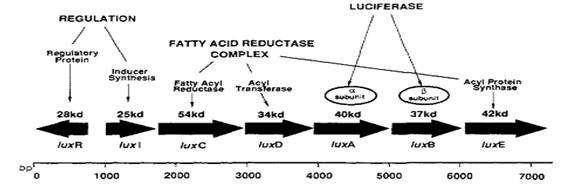
The lux operon
Vibrio fischeri’s lux operon is composed of several genes, all of them involved in bioluminescence and its regulation. This is the structure of Vibrio fischeri’s lux operon:
As it is shown in the diagram luxA and luxB genes, those coding for the luciferase, are placed together into the operon so the strategy was amplify them by means of PCR and then ligate PCR product with a strong constitutive promoter; the exogenously aldehyde added to the strain containing luxAB genes would be enough to trigger the luminescence reaction.
In the first place, we decided to purify genomic DNA from Vibrio fischeri strain MJ11 and then make a PCR with the following primers designed to extract luxAB region:
- Forward primer:Standard iGEM prefix+RBS+Coding Region
CGG AAT TCG CGG CCG CTT CTAG AGGA A ACA GCT ATG AAG TTT GGA AAT ATT TGT TTT TCG TAT CAA CC
- Reverse primer: Standard iGEM suffix+Coding Region
CTG CAG CGG CCG CTA CTA GTA TTA TTA GGG TAG ATT CTT TTC AAT TTT TTG GTT CAA C
YFP protein (LuxY)
LuxY is a gene coding for YFP, a protein that shifts the light emission wavelength of Vibrio fischeri’s luciferase from 484nm (blue) to 534nm (green - yellow); because our system needed a green or yellow emission module we looked in the literature for a protein capable of provoke this phenotype.
The sequence was taken from the following article:
Cloning and expression of the luxY gene from Vibrio fischeri strain Y-1 in Escherichia coli and complete amino acid sequence of the yellow fluorescent protein. Thomas O. Baldwin, Mary L. Treat, S. Colette Daubner Biochemistry 1990 29 (23), 5509-5515
And then the sequence was synthesized by Mr.gene.
This is the composition of the synthesized sequence, “Gene” stands only for luxY coding region.
Preffix+ Promoter + RBS + Gene + Double Terminator + Suffix
Promoter: BBa_J23100
RBS: BBa_B0034
Gene: Vibrio fischeri yellow fluorescent protein (luxY)
Double Terminator: BBa_B0015
See [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360100 LuxY -Part:BBa_K360100-] annotation at the registry for more details.
Second Chassis
Reception module: Green Light cianobacterial Photosensor CcaS/CcaR
OBJECTIVES
METHODOLOGY
Emission module: LuxAB luciferase from vibrio fischeri plus Lumazine protein
OBJECTIVES
- Obtain LuxAB genes from Vibrio fischeri.
- Design Lumazine biobrick, send to synthesise and transfer the construction from Mr. Gene plasmid to pSB1C3 DNA submission backbone.
- To test that the device works as expected. This characterisation will test if Lumazine protein can also be functional working with the Vibrio fischeri luciferase, instead of its native interacting luciferase from Photobacterium phosphoreum.
METHODOLOGY
Lux operon
The strategy to obtain LuxAB genes from Vibrio fischeri was the aforementioned working with the green light emission.
Lumazine
Lumazine protein of Photobacterium phosphoreum (lumP) is a small protein which shifts the wavelength of light emitted by the P.phosphoreum luciferase LuxAB from 495 nm to about 475 nm, we needed it to tune the blue light emission wavelength towards another one closer to that of LovTap system reception.
A version of the lumazine protein was sent to us by Edinburgh Team, due to this construction hadn't been tested we decided to synthesise it, in order to avoid unexpected results for possible errors in the already assembled biobrick from Edinburgh.
This is the composition of the synthesized sequence, “Gene” stands only for lumazine coding region.
Preffix+ Promoter + RBS + Gene + Double Terminator + Suffix
Promoter: BBa_J23119
RBS: BBa_B0034
Gene: Vibrio fischeri yellow fluorescent protein (lumazine)
Double Terminator: BBa_B0015
See [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360122 Lumazine -Part:BBa_K360122-] annotation at the registry for more details.
Third Chassis
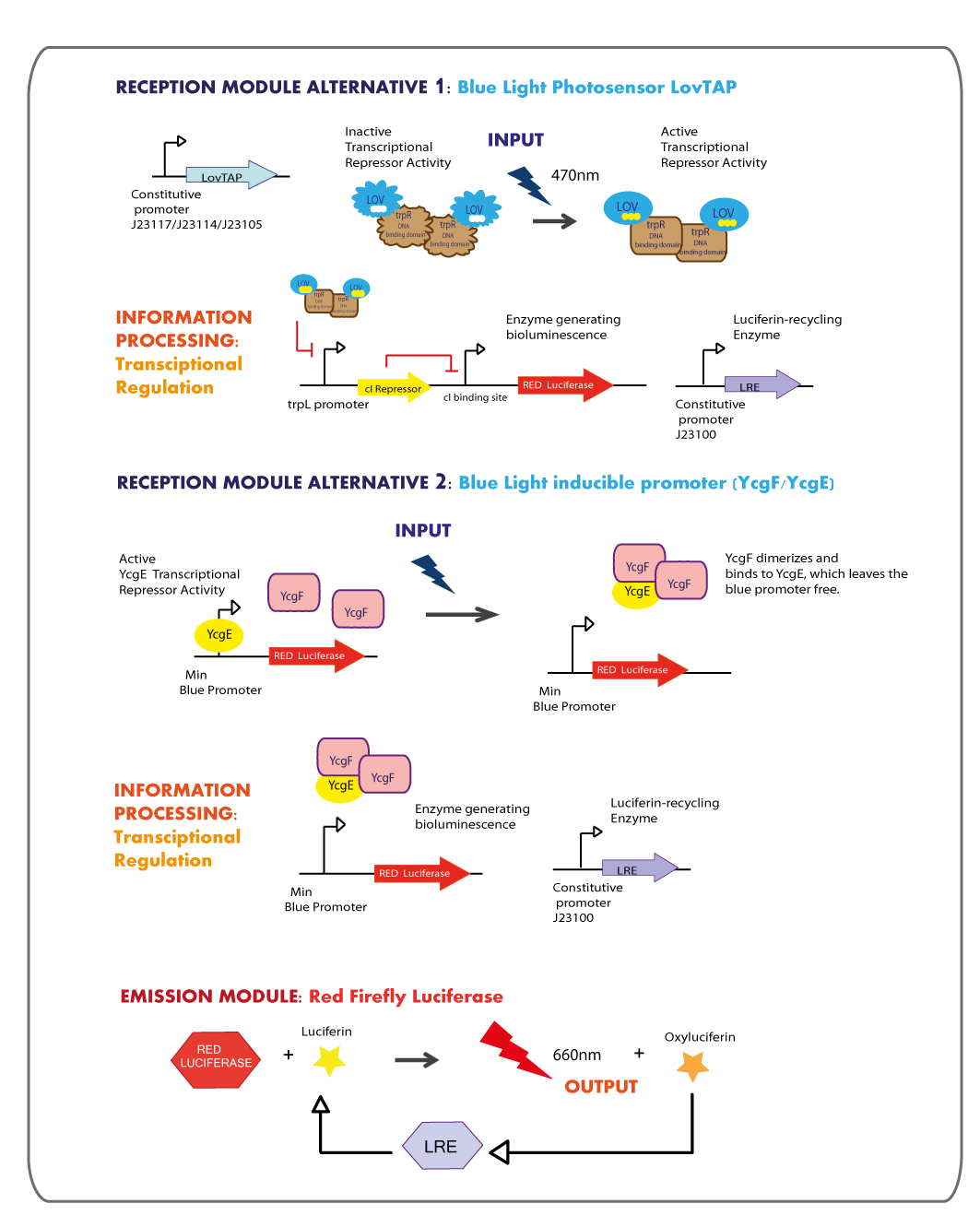
Reception module: Blue Light Photosensor LovTAP and blue light inducible promoter
Working in this chassis, we decided to use as blue light receptors: LovTAP photosensor and the native blue light inducible promoter from E.coli.
LOVTAP
OBJECTIVES
- Construct an improved version of the LovTAP photosensor device under the regulation of different constitutive promoters, due to the previous version of LovTAP designed by 2009 Lausanne team didn´t have a differential behavior under the inverting regulator sensitive to LacI and CAP protein, their results showed that the expression levels of LovTAP didn’t show differences to the induction with IPTG. As well, we included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne. With this mutation LovTAP should react faster and the conformational change should be more stable (the protein stays in the active form for longer, under light induction).
- Transfer the plasmid with the LovTAP synthesised gene from Mr. Gene Plasmid to pSB1C3 DNA submission backbone and ligate the construction to different weak constitutive promoters.
- Characterize LovTAP reporter systems, both repressor and activator activity.
The structure of the device is designed as follows:
METHODOLOGY
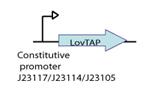
LovTAP design
We decided to synthesize the LovTAP photosensor that in comparison with the Part:BBa_K191003 that is already at the registry, has the following differences:
1. The 2 PstI restriction sites were removed from the coding region of LovTAP.
2. We included a punctual mutation to change the ILE427 by a PHE427, as was proposed by the model results of the team iGEM09_EPF-Lausanne [2]. With this mutation LovTAP should stay in the active form for longer, under light induction.
3. The part does not include a promoter.
4. The RBS was changed from a strong (Part: BBa_B0030) to a medium strength (Part:BBa_B0032).
5. The stop codon tga was changed for two taa.
For more details visit our team [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360121 LovTAP -Part:BBa_K360121-] entry at the registry page.
E.coli Strain Mutant
As the transcriptional response regulated by LovTAP might also be generated by the trpR repressor in E.coli in tryptophan rich conditions , we looked for an Escherichia coli strain mutant in trpR to avoid the cross-talk of the endogenous function of this gene with our system. After a careful look at the literature, we found that Dr. Charles Yanofsky had the mutant, so we sent him an e-mail, asking him to provide us the mutant.
The features of the mutant are:
1.Identification number: CY15001 2.Sex(Hfr,F+,F-,or F'): F-
5 Mutations:
•lamda- (lambda lysogen deletion)
•IN(rrnD-rrnE)1 (Inverts the region between rrnD and rrnE)
•rph-1 (RNase PH )
•tnaA5 (tryptophanase)
•trpR55 (repressor trpR)
LovTAP expression Levels
We got in touch with Dr. Devin Strickland the designer of LovTAP, in order to get some advices. Analyzing some experimental results reported in his dissertation and in the published paper : Light-activated DNA binding in a designed allosteric protein, it seems that at high expression levels of LovTAP, the behavior in the dark versus the light state is the same. So that, the regulation by light becomes not functional, thus we planned to test LovTAP under three different weak constitutive promoters (J23117,J23114 and J23105), expecting that it works well.
trpL promoter
The LovTAP system is composed of two modules, the light-sensitive input module is the LOV2 domain of Avena sativa phototropin 1 (AsLOV2). LOV domains absorb light through a flavin cofactor, photochemically form a covalent bond between the chromophore and a cysteine residue in the protein. The output module is the DNA binding domain of the bacterial transcription factor trp repressor (TrpR). So that, LovTAP activated can bind its operator DNA as a homodimer thus repressing transcription.
The DNA operator region where trp repressor binds was annotated wrongly in the 2009 EPF-Lausanne’s wiki as trpO promoter, but when searching for it in RegulonDB a very well annotated Escherichia coli transcriptional regulation data base, we found that the actually region where trp repressor binds is trpL promoter not trpO.
Knowing that we synthesized only trpLp’s functional elements as an oligonucleotide to introduce by PCR into a selected plasmid as follows:
Primer trpL reverse (5'->3'): NheI site + trpL promoter + XbaI site + EcoRI site:
TTGCTAGCGTGAACTTGCGTACTAGTTAACTAGTTCGATGATTAATTGTCAACAGCCTCTAGAAGCGGCCGCGAATTC
The primer is the reverse complementary of that sequence so we can use it as a reverse primer, which we used along with a suffix forward to introduce the trpL promoter into pSB1C3 plasmid by PCR.
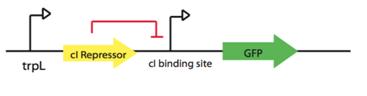
LovTAP Reporter systems
As we are interested in characterize LovTAP activator and repressor activity. We have designed the following constructions.
- LovTAP repressor activity: Reporter system
1.trpL promoter fused to GFP protein:Part:BBa_E0240
2.trpL promoter fused to RFP protein: Part: BBa_E1010
- LovTAP activator activity: Reporter system
trpL promoter fused to lambda Repressor cI: Part:BBa_P0451 + Part:BBa_K098991 regulating GFP protein:Part:BBa_E0240.
YcgF/YcgE BLUE RECEPTION SYSTEM
OBJECTIVES
The main goal for this section of the project was to implement a system for blue light reception using the YcgF/YcgE sytem, naturally present in certain strains of Escherichia coli.
This reception system would be coupled both to a reporter gene (i.e. GFP) and, for the purposes of our project, a luciferase gene.
METHODOLOGY
We used a modified version of the YcgF/YcgE system for blue light reception (previously reported by the K.U. Leuven 2009 Team), the modification consists in the reduction of the promoter region to 50 bp.
Originally, we tried to amplify the complete promoter region BBa_238013 (86 bp) from genomic DNA of E. coli K12 by PCR but we failed in several trials. So we decided to synthesize the promoter in a primer and then insert it into pSB4A5 by PCR. Because of lenght limitations in primer synthesis we reduced the promoter region to 50 bp.
The reduced promoter retains the fundamental parts of the original one, these are the -35 and -10 box, the spacer between them, the inverted repeat 1 and 2 (inverted regions are the binding sites for the YcgE repressor) and the transcription start site. We registered this Minimum Blue Light Receptor Promoter as BBa_K360041.
In order to test the functionality of our Minimum Blue Promoter we succesfully ligated it to our Strong RBS BBa_K360031 and the GFP BBa_E0040.
Emission module: Red Firefly Luciferase
The red light emitter system that we decided to use is the firefly luciferase, this luciferase usually emits green light but with a punctual mutation we can shift the emission spectrum from green to red.
OBJECTIVES
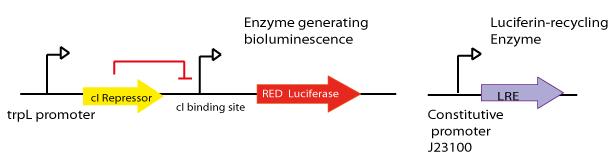
- The final aim was the construction of the red light emission device, using the promoter region regulated by LovTAP with the red light emitting luciferase coupled to the Luciferin Regenerating Enzyme (LRE). The structure of the device is designed as follows:
- Design LRE biobrick and transfer the plasmid with the synthesised gene from Mr. Gene Plasmid to pSB1C3 DNA submission backbone and assemble it to a constitutive promoter.
- To test that the device works as expected.
METHODOLOGY
Mutated Firefly Luciferase
The original intention was to use the Photinus pyralis luciferase biobrick (BBa_I712019) as a base and to increase its efficiency by using the primer BBa_K360114 (as registered) due to previous design notes as referred in the part design section of this briobrick. After achieving this, a punctual mutation S284T (also known as S851T) was induced to change its emission spectrum from green to red by means of a PCR with special primers (which are also referred in the biobrick part design). The biobrick name is BBa_K360115; unfortunately, we were never able to find out why it was not functional.
Click Beetle Luciferase
By the middle of the summer, we decided to try the luciferase from another organism: Click Beetle, specifically the Promega Chroma-Luc™ Reporter Vectors pCBG99-Basic Vector Restriction Sites and the pCBR-Basic Vector Restriction Sites, where the first one has a green emission and the second one red. The intention was to work with these vectors as a means of having a previously-known-to-be functional luciferase, so that once the LRE biobrick was ready, characterization could be feasible. The first step was to extract the coding sequence of the luciferases from these vectors by means of PCR and also to standardize them by adding the prefix and suffix with the following primers:
Forward Click Beetle luciferase
GAATTCGCGGCCGCTTCTAGAGATTAAAGAGGAGAAAATGGTGAAGCGTGAGAAAAAT
Reverse Click Beetle luciferase
CTGCAGCGGCCGCTACTAGTATTATTAACCGCCGGCCTTCT
The second step was to ligate it into the BBa_J61002 plasmid with a J23101 promoter, and to transform it, having as an output several colonies which were then grown overnight to check whether the luciferases showed any brightness. The protocol followed for the assay is mentioned in BBa_K216015 biobrick, in the Experience part. In this first assay, no luminometer was used; so eventhough we added 1μL of 100mM luciferin and waited in the dark room for ~30min taking pictures with a SLR camera with high ISO (~400), for about 30secs to 1 min exposure, nothing was observed. After the initial disappointment, Mariana talked to Cambridge team and they help us ever since then by sending us some of their constructions (BBa_K325909, BBa_K325109, BBa_K325209, and 2 other biobricks).
LRE design
Luciferin Regenerating Enzyme (LRE) was first reported by Gomi, K. and Kajiyama, N. [(2001) Oxyluciferin, a Luminescence Product of Firefly Luciferase, Is Enzymatically Regenerated into Luciferin. The Journal of Biological Chemistry, Vol. 276, No. 39. This enzyme proved to recycle the luciferase product oxyluciferin. Our goal here was to couple both, luciferase and LRE, to be able to make the construction autonomous after the first and single input of luciferin. We sent to synthesize the LRE enzyme.
For more details see [http://partsregistry.org/wiki/index.php?title=Part:BBa_K360113 LRE -Part:BBa_K360113-] entry at the Registry page.iGEM
iGEM is the International Genetically Engineered Machines Competition, held each year at MIT and organized with support of the Parts Registry. See more here.Synthetic Biology
This is defined as attempting to manipulate living objects as if they were man-made machines, specifically in terms of genetic engineering. See more here.Genomics
We are students on the Genomic Sciences program at the Center for Genomic Sciences of the National Autonomous University of Mexico, campus Morelos. See more here.This site is best viewed with a Webkit based Browser (eg: Google's Chrome, Apple's Safari),
Trident based (Microsoft's Internet Explorer) or Presto based (Opera) are not currently supported. Sorry.
 "
"