Team:HokkaidoU Japan/Notebook/August18
From 2010.igem.org
(Difference between revisions)
(→Digestion (Xba I, Pst I)) |
|||
| (26 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:HokkaidoU_Japan}}<div class="linkbar"><div class="prev">[[Team:HokkaidoU_Japan/Notebook/August17|August 17]]</div>[[Team:HokkaidoU_Japan/Notebook|Notebook]]<div class="next">[[Team:HokkaidoU_Japan/Notebook/August19|August 19]]</div></div> | {{Template:HokkaidoU_Japan}}<div class="linkbar"><div class="prev">[[Team:HokkaidoU_Japan/Notebook/August17|August 17]]</div>[[Team:HokkaidoU_Japan/Notebook|Notebook]]<div class="next">[[Team:HokkaidoU_Japan/Notebook/August19|August 19]]</div></div> | ||
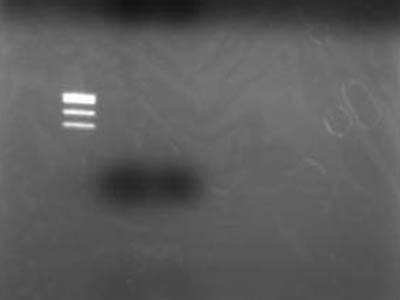
| - | =RBS | + | =RBS digestion: Revenge= |
[[Image:HokkaidoU Japan 20100818a.JPG|200px|right|thumb|]] | [[Image:HokkaidoU Japan 20100818a.JPG|200px|right|thumb|]] | ||
{|style="text-align: center" class="protocol" | {|style="text-align: center" class="protocol" | ||
| + | !Reagent | ||
| + | !Amount | ||
|- | |- | ||
|1-2M | |1-2M | ||
| Line 22: | Line 24: | ||
|1 uL | |1 uL | ||
|- | |- | ||
| - | |style="border-top:1px solid # | + | |style="border-top:1px solid #996"|'''Total''' |
| - | |style="border-top:1px solid # | + | |style="border-top:1px solid #996"|'''20 uL''' |
|} | |} | ||
| - | + | ->Incubated at 37C for 60 min | |
| - | * 4 uL 6x Sample | + | * Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed |
| - | + | ->Extracted for gel <br> | |
| - | + | ->Electrophoresed 10 uL of Extracted DNA | |
| + | * Used [https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png TSUDA Marker 1] | ||
| - | === | + | ===Failure=== |
| - | * | + | * After better inspection of Kit specs it came to lite that retreavel rate for 50 bp and less is 26% |
| + | ** RBS is just quite small when cut | ||
=Ligation= | =Ligation= | ||
| - | === | + | ===Preparation of DNA Solution for Ligation=== |
| - | {|style="text-align:center;" class="protocol" | + | {|border="1px" style="text-align:center;" class="protocol" |
|- | |- | ||
| - | + | !Part | |
| - | + | !By comparison to[https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png λ/''Hin''d III]<br>(ng/10 uL) | |
| - | + | !ng/uL | |
| - | + | !ratio | |
| - | + | !size(bp) | |
| - | + | !required(ng) | |
| - | + | !used(uL) | |
|- | |- | ||
|Vector | |Vector | ||
| Line 49: | Line 53: | ||
|5 ng/uL | |5 ng/uL | ||
|1 | |1 | ||
| - | | | + | |2996 bp |
|10 ng | |10 ng | ||
|2 uL | |2 uL | ||
| Line 61: | Line 65: | ||
|0.3 uL | |0.3 uL | ||
|- | |- | ||
| - | | | + | |terminator |
|5 ng/10 uL | |5 ng/10 uL | ||
|0.5 ng/uL | |0.5 ng/uL | ||
| Line 69: | Line 73: | ||
|4 uL | |4 uL | ||
|- | |- | ||
| - | |style="border-top:1px solid # | + | |style="border-top:1px solid #996; text-align:right;" colspan="6"|'''Total''' |
| - | |style="border-top:1px solid # | + | |style="border-top:1px solid #996;"|'''6.3 uL''' |
|} | |} | ||
| - | ===Ligation | + | ===Ligation and [[Team:HokkaidoU_Japan/Protocols|Transformation]]=== |
{|style="text-align:center;" class="protocol" | {|style="text-align:center;" class="protocol" | ||
| + | !Reagent | ||
| + | !Amount | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| Line 84: | Line 90: | ||
|T4 ligase | |T4 ligase | ||
|1 uL | |1 uL | ||
| + | |- | ||
| + | |style="border-top:1px solid #996;"|'''Total''' | ||
| + | |style="border-top:1px solid #996;"|'''13.6 uL''' | ||
|} | |} | ||
| - | * | + | * Incubated at 16C for 30 min |
| - | * competent cell | + | * Transformation: Added all to 50 uL of competent cell |
| - | * | + | * Incubated at 0C for 30 min |
| - | * | + | * Heat shocked at 42C for 60 sec |
* 5 min on ice | * 5 min on ice | ||
| - | * | + | * Added 100 uL of LB |
| - | * | + | * Incubated at 37C for 120 min |
| - | * | + | * Spread onto the LBC plate |
| - | * | + | * Incubated at 37C for 20 hrs |
| - | + | ||
| - | =RBS | + | =RBS Retry= |
| - | + | ==Digestion== | |
{|style="text-align: center" class="protocol" | {|style="text-align: center" class="protocol" | ||
| + | !Reagent | ||
| + | !Amount | ||
|- | |- | ||
|(RBS)1-2M | |(RBS)1-2M | ||
| Line 119: | Line 129: | ||
|1 uL | |1 uL | ||
|- | |- | ||
| - | |style="border-top:1px solid # | + | |style="border-top:1px solid #996"|'''Total''' |
| - | |style="border-top:1px solid # | + | |style="border-top:1px solid #996"|'''20 uL''' |
|} | |} | ||
| - | + | ->Incubated at 37C for 60 min | |
| - | == | + | ==[[Team:HokkaidoU_Japan/Protocols|Ethanol Precipication]]== |
| - | * 2 | + | * Added 2 uL of sodium acetate(3 M) |
| - | * 44 | + | * Added 44 uL of Ethanol |
| - | * | + | * Transfered to 1.5 mL tube and frozen with liquid nitrogen |
| - | * | + | * Melted and centrifuged at 15000 rpm, 4C for 5 min |
| - | * | + | * Transfered supernatant to another tube |
| - | * 100 | + | ** Driven by precaution centrifuged the supernatant and discarded it's supernatant |
| - | * | + | * Rinsed the tube walls with 100 uL of 70% Ethanol and centrifuged at 15000 rpm, 4C for 5 min |
| - | * | + | * Discarded the supertenant and dried via vacuum desiccator |
| + | * Melted in 5 uL of TE | ||
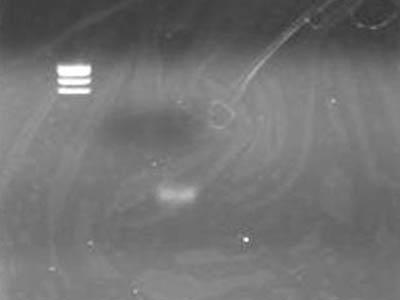
| - | == | + | ==Electrophoresis== |
| - | [[Image:HokkaidoU Japan 20100818b.JPG|200px|right|thumb|]] | + | [[Image:HokkaidoU Japan 20100818b.JPG|200px|right|thumb| Electrophoresis after Ethanol presipication]] |
| - | * | + | * Added 1 uL of 6x SB to DNA Solution of 1 uL |
| - | * | + | * Did the same to supernatant retreaved for precaution |
{| class="protocol" | {| class="protocol" | ||
|- | |- | ||
| - | | | + | |'''Lane''' |
| - | | | + | |'''DNA''' |
|- | |- | ||
|2 | |2 | ||
| - | |TSUDA Marker 1 | + | |[https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png TSUDA Marker 1] |
|- | |- | ||
|3 | |3 | ||
| - | | | + | |supernatant |
|- | |- | ||
|4 | |4 | ||
|DNA solution | |DNA solution | ||
|} | |} | ||
| - | * DNA | + | * DNA solution band is visible |
| + | ** Looks OK | ||
Latest revision as of 13:28, 27 October 2010
RBS digestion: Revenge
| Reagent | Amount |
|---|---|
| 1-2M | 10 uL |
| DW | 4 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
->Incubated at 37C for 60 min
- Added 4 uL of 6x Sample Buffer making it total of 12 uL per lane and electrophoresed
->Extracted for gel
->Electrophoresed 10 uL of Extracted DNA
- Used TSUDA Marker 1
Failure
- After better inspection of Kit specs it came to lite that retreavel rate for 50 bp and less is 26%
- RBS is just quite small when cut
Ligation
Preparation of DNA Solution for Ligation
| Part | By comparison toλ/Hind III (ng/10 uL) | ng/uL | ratio | size(bp) | required(ng) | used(uL) |
|---|---|---|---|---|---|---|
| Vector | 50 ng/10 uL | 5 ng/uL | 1 | 2996 bp | 10 ng | 2 uL |
| RFP | 250 ng/10 uL | 25 ng/uL | 2 | 700 bp | 7 ng | 0.3 uL |
| terminator | 5 ng/10 uL | 0.5 ng/uL | 2 | 200 bp | 2 ng | 4 uL |
| Total | 6.3 uL | |||||
Ligation and Transformation
| Reagent | Amount |
|---|---|
| DNA solution | 6.3 uL |
| Ligation solution | 6.3 uL |
| T4 ligase | 1 uL |
| Total | 13.6 uL |
- Incubated at 16C for 30 min
- Transformation: Added all to 50 uL of competent cell
- Incubated at 0C for 30 min
- Heat shocked at 42C for 60 sec
- 5 min on ice
- Added 100 uL of LB
- Incubated at 37C for 120 min
- Spread onto the LBC plate
- Incubated at 37C for 20 hrs
RBS Retry
Digestion
| Reagent | Amount |
|---|---|
| (RBS)1-2M | 10 uL |
| DW | 4 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
->Incubated at 37C for 60 min
Ethanol Precipication
- Added 2 uL of sodium acetate(3 M)
- Added 44 uL of Ethanol
- Transfered to 1.5 mL tube and frozen with liquid nitrogen
- Melted and centrifuged at 15000 rpm, 4C for 5 min
- Transfered supernatant to another tube
- Driven by precaution centrifuged the supernatant and discarded it's supernatant
- Rinsed the tube walls with 100 uL of 70% Ethanol and centrifuged at 15000 rpm, 4C for 5 min
- Discarded the supertenant and dried via vacuum desiccator
- Melted in 5 uL of TE
Electrophoresis
- Added 1 uL of 6x SB to DNA Solution of 1 uL
- Did the same to supernatant retreaved for precaution
| Lane | DNA |
| 2 | TSUDA Marker 1 |
| 3 | supernatant |
| 4 | DNA solution |
- DNA solution band is visible
- Looks OK
 "
"