Team:HokkaidoU Japan/Notebook/August30
From 2010.igem.org
(Difference between revisions)
(New page: {{Template:HokkaidoU_Japan}}) |
|||
| (23 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{Template:HokkaidoU_Japan}} | + | {{Template:HokkaidoU_Japan}}<div class="linkbar"><div class="prev">[[Team:HokkaidoU_Japan/Notebook/August27|August 27]]</div>[[Team:HokkaidoU_Japan/Notebook|Notebook]]<div class="next">[[Team:HokkaidoU_Japan/Notebook/August31|August 31]]</div></div> |
| + | |||
| + | =Digestion of pUC119 by EcoR I, Pst I= | ||
| + | |||
| + | {|border="1" style="text-align:center;" class="protocol" | ||
| + | |- | ||
| + | || | ||
| + | |style="border-bottom:1px;"|- | ||
| + | |style="border-bottom:1px;"|EcoR I | ||
| + | |style="border-bottom:1px;"|Pst I | ||
| + | |style="border-bottom:1px;"|E, P | ||
| + | |style="border-bottom:1px;"|Old EcoR I | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |- | ||
| + | |DW | ||
| + | |17 uL | ||
| + | |14 uL | ||
| + | |14 uL | ||
| + | |13 uL | ||
| + | |14 uL | ||
| + | |- | ||
| + | |10x M buffer | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |- | ||
| + | |0.1% BSA | ||
| + | |- | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |- | ||
| + | |EcoR I | ||
| + | |- | ||
| + | |1 uL | ||
| + | |- | ||
| + | |1 uL | ||
| + | |''1 uL'' | ||
| + | |- | ||
| + | |Pst I | ||
| + | |- | ||
| + | |- | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |- | ||
| + | |- | ||
| + | |style="border-top:1px;border-right:1px;"|'''Total''' | ||
| + | ||'''20 uL''' | ||
| + | ||'''20 uL''' | ||
| + | ||'''20 uL''' | ||
| + | ||'''20 uL''' | ||
| + | ||'''20 uL''' | ||
| + | |} | ||
| + | |||
| + | →Incubated at 37C for 60 min | ||
| + | →Electrophoresed 2 uL for confirmation | ||
| + | * There were no bands, forgot to add DNA | ||
| + | * Reused the remaining 18 uL of digestion solution by adding 1 uL of ADW and 1uL of DNA | ||
| + | →Incubated at 37C for 60 min<br> | ||
| + | →Electrophoresed 2 uL of each solution(+ 0.4 uL 6x SB) | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
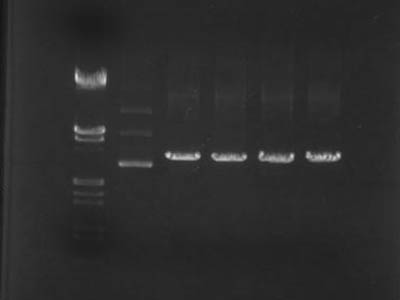
| + | [[Image:HokkaidoU Japan 20100830b.jpg|200px|right|thumb|Electrophoresis of digestion products]] | ||
| + | |||
| + | {| class="protocol" | ||
| + | |'''Lane''' | ||
| + | |'''DNA''' | ||
| + | |- | ||
| + | |2 | ||
| + | |[https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png λ/''Hin''dIII, EcoR I](4 uL used) | ||
| + | |- | ||
| + | |3 | ||
| + | |Undigested | ||
| + | |- | ||
| + | |4 | ||
| + | |EcoR I | ||
| + | |- | ||
| + | |5 | ||
| + | |Pst I | ||
| + | |- | ||
| + | |6 | ||
| + | |EcoR I + Pst I | ||
| + | |- | ||
| + | |7 | ||
| + | |EcoR I (used old enzyme to check it's activity) | ||
| + | |} | ||
| + | * In lane 3 monomers, dimers and trimers of plasmid were visible . | ||
| + | * From lanes 4 through 7 it's visible that DNA digestion wasn't satisfactory | ||
| + | ** Because plasmid became linear it's was slower than super-coiled one's | ||
| + | |||
| + | ==Digestion of parts PCRed using digestion visualization primers== | ||
| + | {|style="text-align:center;" class="protocol" | ||
| + | |- | ||
| + | |style="border-right:1px solid #996; border-bottom:1px solid #996;"| | ||
| + | |colspan="4" style="border-right:1px solid #996; border-bottom:1px solid #996;"|RBS | ||
| + | |colspan="4" style="border-right:1px solid #996; border-bottom:1px solid #996;"|double terminator | ||
| + | |colspan="4" style="border-bottom:1px solid #996;"|GFP | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|DNA | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |style="border-right:1px solid #996;"|1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |style="border-right:1px solid #996;"|1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|DW | ||
| + | |17 uL | ||
| + | |14 uL | ||
| + | |14 uL | ||
| + | |style="border-right:1px solid #996;"|13 uL | ||
| + | |17 uL | ||
| + | |14 uL | ||
| + | |14 uL | ||
| + | |style="border-right:1px solid #996;"|13 uL | ||
| + | |17 uL | ||
| + | |14 uL | ||
| + | |14 uL | ||
| + | |13 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|10x M buffer | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |style="border-right:1px solid #996;"|2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |style="border-right:1px solid #996;"|2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|0.1% BSA | ||
| + | |- | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |style="border-right:1px solid #996;"|2 uL | ||
| + | |- | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |style="border-right:1px solid #996;"|2 uL | ||
| + | |- | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |2 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|EcoR I | ||
| + | |- | ||
| + | |1 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|1 uL | ||
| + | |- | ||
| + | |1 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|1 uL | ||
| + | |- | ||
| + | |1 uL | ||
| + | |- | ||
| + | |1 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996;"|Pst I | ||
| + | |- | ||
| + | |- | ||
| + | |1 uL | ||
| + | |style="border-right:1px solid #996;"|1 uL | ||
| + | |- | ||
| + | |- | ||
| + | |1 uL | ||
| + | |style="border-right:1px solid #996;"|1 uL | ||
| + | |- | ||
| + | |- | ||
| + | |1 uL | ||
| + | |1 uL | ||
| + | |- | ||
| + | |style="border-right:1px solid #996; border-top:1px solid #996;"|Total | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996; border-right:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996; border-right:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |style="border-top:1px solid #996;"|20 uL | ||
| + | |} | ||
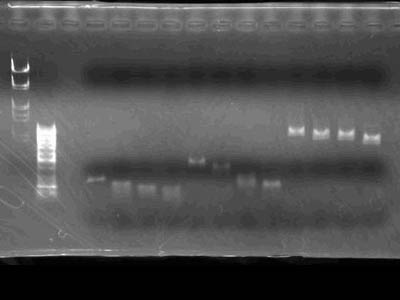
| + | [[Image:HokkaidoU Japan 20100830a.jpg|200px|right|thumb|Electrophoresis of digestion products]] | ||
| + | →Incubated at 37C for 60 min | ||
| + | ===Electrophoresis=== | ||
| + | * Markers used [https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png λ/''Hin''dIII, EcoR I] and 50bp ladder | ||
| + | * Was obvious that parts were cut as intended | ||
Latest revision as of 07:59, 27 October 2010
Digestion of pUC119 by EcoR I, Pst I
| - | EcoR I | Pst I | E, P | Old EcoR I | |
| DNA solution | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL |
| DW | 17 uL | 14 uL | 14 uL | 13 uL | 14 uL |
| 10x M buffer | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL |
| 0.1% BSA | - | 2 uL | 2 uL | 2 uL | 2 uL |
| EcoR I | - | 1 uL | - | 1 uL | 1 uL |
| Pst I | - | - | 1 uL | 1 uL | - |
| Total | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL |
→Incubated at 37C for 60 min →Electrophoresed 2 uL for confirmation
- There were no bands, forgot to add DNA
- Reused the remaining 18 uL of digestion solution by adding 1 uL of ADW and 1uL of DNA
→Incubated at 37C for 60 min
→Electrophoresed 2 uL of each solution(+ 0.4 uL 6x SB)
Electrophoresis
| Lane | DNA |
| 2 | λ/HindIII, EcoR I(4 uL used) |
| 3 | Undigested |
| 4 | EcoR I |
| 5 | Pst I |
| 6 | EcoR I + Pst I |
| 7 | EcoR I (used old enzyme to check it's activity) |
- In lane 3 monomers, dimers and trimers of plasmid were visible .
- From lanes 4 through 7 it's visible that DNA digestion wasn't satisfactory
- Because plasmid became linear it's was slower than super-coiled one's
Digestion of parts PCRed using digestion visualization primers
| RBS | double terminator | GFP | ||||||||||
| DNA | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL |
| DW | 17 uL | 14 uL | 14 uL | 13 uL | 17 uL | 14 uL | 14 uL | 13 uL | 17 uL | 14 uL | 14 uL | 13 uL |
| 10x M buffer | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL |
| 0.1% BSA | - | 2 uL | 2 uL | 2 uL | - | 2 uL | 2 uL | 2 uL | - | 2 uL | 2 uL | 2 uL |
| EcoR I | - | 1 uL | - | 1 uL | - | 1 uL | - | 1 uL | - | 1 uL | - | 1 uL |
| Pst I | - | - | 1 uL | 1 uL | - | - | 1 uL | 1 uL | - | - | 1 uL | 1 uL |
| Total | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL | 20 uL |
→Incubated at 37C for 60 min
Electrophoresis
- Markers used λ/HindIII, EcoR I and 50bp ladder
- Was obvious that parts were cut as intended
 "
"