Team:HokkaidoU Japan/Notebook/August16
From 2010.igem.org
(Difference between revisions)
(→Restriction Enzyme Digestion of the vector (EcoR I, Pst I)) |
|||
| (8 intermediate revisions not shown) | |||
| Line 38: | Line 38: | ||
!Amount | !Amount | ||
|- | |- | ||
| - | |1-1A | + | |[[Team:HokkaidoU_Japan/Parts#BioBricks|1-1A]] |
|1 uL | |1 uL | ||
|- | |- | ||
| Line 60: | Line 60: | ||
===Control=== | ===Control=== | ||
* Added 1 uL of 6x Sample Buffer to 1 uL of pUC119 | * Added 1 uL of 6x Sample Buffer to 1 uL of pUC119 | ||
| - | * Also added 1 uL of 6x Sample Buffer to 1 uL of 1-1A | + | * Also added 1 uL of 6x Sample Buffer to 1 uL of [[Team:HokkaidoU_Japan/Parts#BioBricks|1-1A]] |
** Incubated at 37C for 60 min | ** Incubated at 37C for 60 min | ||
| Line 74: | Line 74: | ||
!DNA | !DNA | ||
|- | |- | ||
| - | | | + | |1 |
| - | |[https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png | + | |[https://2010.igem.org/Image:HokkaidoU_Pictures_DNA_Marker.png λ/''Hin''dIII] |
|- | |- | ||
| - | | | + | |2 |
|pUC119 control | |pUC119 control | ||
|- | |- | ||
| - | | | + | |3 |
| - | |1-1A control | + | |[[Team:HokkaidoU_Japan/Parts#BioBricks|1-1A]] control |
|- | |- | ||
| - | | | + | |4 |
|pUC119 + EcoR I | |pUC119 + EcoR I | ||
|- | |- | ||
| - | | | + | |5 |
|pUC119 + Xba I | |pUC119 + Xba I | ||
|- | |- | ||
| - | | | + | |6 |
| - | |1-1A + Spe I | + | |[Team:HokkaidoU_Japan/Parts#BioBricks|[1-1A]] + Spe I |
|- | |- | ||
| - | | | + | |7 |
|pUC119 + Pst I | |pUC119 + Pst I | ||
|- | |- | ||
| - | | | + | |8 |
|Empty | |Empty | ||
|} | |} | ||
| Line 136: | Line 136: | ||
!Amount | !Amount | ||
|- | |- | ||
| - | |1-2M | + | |[[Team:HokkaidoU_Japan/Parts#BioBricks|1-2M]] |
|2.5 uL | |2.5 uL | ||
|- | |- | ||
| Line 158: | Line 158: | ||
|} | |} | ||
</div> | </div> | ||
| - | <div style="float:left;"> | + | <div style="float:left; margin-left:50px;"> |
'''Terminator''' | '''Terminator''' | ||
{|style="text-align: center;" class="protocol" | {|style="text-align: center;" class="protocol" | ||
| Line 165: | Line 165: | ||
!Amount | !Amount | ||
|- | |- | ||
| - | |1-23L | + | |[[Team:HokkaidoU_Japan/Parts#BioBricks|1-23L]] |
|1.5 uL | |1.5 uL | ||
|- | |- | ||
| Line 189: | Line 189: | ||
<div style="clear:both;"></div> | <div style="clear:both;"></div> | ||
| - | = | + | =Restriction Enzyme (EcoR I, Spe I) Digestion of the Parts= |
<div style="float:left;"> | <div style="float:left;"> | ||
'''Heat shock promotor''' | '''Heat shock promotor''' | ||
| Line 197: | Line 197: | ||
!Amount | !Amount | ||
|- | |- | ||
| - | |3-1E | + | |[[Team:HokkaidoU_Japan/Parts#BioBricks|3-1E]] |
|5 uL | |5 uL | ||
|- | |- | ||
| Line 219: | Line 219: | ||
|} | |} | ||
</div> | </div> | ||
| - | <div style="float:left;"> | + | <div style="float:left; margin-left:50px;"> |
'''RFP''' | '''RFP''' | ||
{|style="text-align: center;" class="protocol" | {|style="text-align: center;" class="protocol" | ||
| Line 226: | Line 226: | ||
!Amount | !Amount | ||
|- | |- | ||
| - | |1-18F | + | |[[Team:HokkaidoU_Japan/Parts#BioBricks|1-18F]] |
|5 uL | |5 uL | ||
|- | |- | ||
| Line 249: | Line 249: | ||
</div> | </div> | ||
<div style="clear:both;"></div> | <div style="clear:both;"></div> | ||
| - | * | + | * It was obvious after restriction enzyme activity check that Spe I was week, so additional 0.7 uL was added |
Latest revision as of 07:31, 27 October 2010
Restriction Enzyme Activity Check
Restriction Enzyme Digestion
EcoR I, Xba I, Pst I
| Reagent | Amount |
|---|---|
| pUC119 | 1 uL |
| DW | 7 uL |
| 10x M Buffer | 1 uL |
| Restriction Enzyme | 1 uL |
| Total | 10 uL |
- Made solution for each EcoR I, Xba I, Pst I
- incubated at 37C for 60 min
Spe I
| Reagent | Amount |
|---|---|
| 1-1A | 1 uL |
| DW | 7 uL |
| 10x M Buffer | 1 uL |
| Restriction Enzyme | 1 uL |
| Total | 10 uL |
- pUC119 doesn't have Spe I restriction sites, so we used mini preped plasmid with biobrick insert
- incubated at 37C for 60 min
Control
- Added 1 uL of 6x Sample Buffer to 1 uL of pUC119
- Also added 1 uL of 6x Sample Buffer to 1 uL of 1-1A
- Incubated at 37C for 60 min
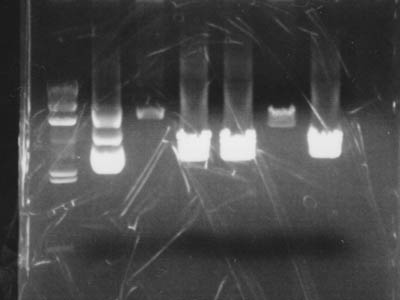
Electrophoresis
Added 2 uL of 6x Sample Buffer to digested solution and performed electrophoresis
| Lane | DNA |
|---|---|
| 1 | λ/HindIII |
| 2 | pUC119 control |
| 3 | 1-1A control |
| 4 | pUC119 + EcoR I |
| 5 | pUC119 + Xba I |
| 6 | [1-1A]] + Spe I |
| 7 | pUC119 + Pst I |
| 8 | Empty |
Restriction Enzyme (EcoR I, Pst I) Digestion of the vector
| Reagent | Amount |
|---|---|
| pSB1C3 | 4 uL |
| DW | 10 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| EcoR I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
Restriction Enzyme (Xba I, Pst I) Digestion of the Parts
RBS
| Reagent | Amount |
|---|---|
| 1-2M | 2.5 uL |
| DW | 11.5 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
Terminator
| Reagent | Amount |
|---|---|
| 1-23L | 1.5 uL |
| DW | 12.5 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| Xba I | 1 uL |
| Pst I | 1 uL |
| Total | 20 uL |
Restriction Enzyme (EcoR I, Spe I) Digestion of the Parts
Heat shock promotor
| Reagent | Amount |
|---|---|
| 3-1E | 5 uL |
| DW | 9.7 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| EcoR I | 1 uL |
| Spe I | 0.3 uL |
| Total | 20 uL |
RFP
| Reagent | Amount |
|---|---|
| 1-18F | 5 uL |
| DW | 9.7 uL |
| 10x M Buffer | 2 uL |
| BSA | 2 uL |
| EcoR I | 1 uL |
| Spe I | 0.3 uL |
| Total | 20 uL |
- It was obvious after restriction enzyme activity check that Spe I was week, so additional 0.7 uL was added
 "
"