Team:Mexico-UNAM-CINVESTAV/Notebook/Week Four
From 2010.igem.org
(→Wenesday) |
|||
| (32 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Mexico-UNAM-CINVESTAV-HEADER}} | {{Mexico-UNAM-CINVESTAV-HEADER}} | ||
| + | __NOTOC__ | ||
| - | = Week 4 | + | |
| + | = Week #4= | ||
| + | |||
| + | =27th September - 01 October 2010 = | ||
==''Monday''== | ==''Monday''== | ||
| - | ==='''Well we are stuck with the vector | + | ==='''Well...we are stuck with the vector. This week we have to get it.'''=== |
| - | *'''We have | + | *'''We have achieved a nano spectrophotometer and readings are below:''' |
| Line 69: | Line 73: | ||
| - | *'''Yep our trouble is the | + | *'''Yep, our trouble is the miniprep method''' |
| + | |||
| + | '''we have a low concentration of vector plasmid.''' | ||
| + | |||
| + | '''We have to work an try to get more plasmid and used diluted PCR products''' | ||
| + | |||
| + | '''is not possible achieve ligations with this vector an insert ratio.''' | ||
| + | |||
| + | '''Our advisor proposed use low Melting agarose''' | ||
| + | |||
| + | '''to extract the plasmid from the gel.''' | ||
| + | |||
| + | |||
| + | ==''Tuesday''== | ||
| + | |||
| + | ==='''We ran with a low meelting agarose and cut off the band '''=== | ||
| + | ==='''we precipitated using alchol and salts in order '''=== | ||
| + | ==='''to get enough vector to do the ligations.'''=== | ||
| + | |||
| + | |||
| + | [[Image:Imagen5.gif]] | ||
| + | |||
| + | |||
| + | |||
| + | ==''Wednesday''== | ||
| + | |||
| + | ==='''Plan'''=== | ||
| + | |||
| + | *'''We set up all to do the ligations.''' | ||
| + | *'''We digested both vector and insert with ''EcoR''I and ''Pst''I .''' | ||
| + | *'''We ran a low melting agarose gel 1 hour at 80 volts, then cut off the band from gel.''' | ||
| + | |||
| + | '''At Claudia's lab we tried three two different kits to recover DNA from gel.''' | ||
| + | |||
| + | *'''Mobio ultra clean Microbial DNA kit''' | ||
| + | *'''Axygen.....''' | ||
| + | *''' | ||
| + | |||
| + | '''The digestions were done with following amounts.''' | ||
| + | |||
| + | {| border="1" | ||
| + | |- | ||
| + | |DNA | ||
| + | |20μl | ||
| + | |- | ||
| + | |Buffer NB2 | ||
| + | |3μl | ||
| + | |- | ||
| + | |EcoRI | ||
| + | |2μl | ||
| + | |- | ||
| + | |PstI | ||
| + | |2μl | ||
| + | |- | ||
| + | |H2O | ||
| + | |2.4μl | ||
| + | |- | ||
| + | |BSA | ||
| + | |0.6μl | ||
| + | |- | ||
| + | |Total | ||
| + | |30μl | ||
| + | |} | ||
| + | |||
| + | ==''Thursday''== | ||
| + | |||
| + | ==='''Plan'''=== | ||
| + | |||
| + | '''We then proceed to diluted PCR fragments''' | ||
| + | |||
| + | '''For this we did the following operations:''' | ||
| + | |||
| + | '''Poner las fórmulas usadas en un formato bonito''' | ||
| + | |||
| + | '''We did the ligations using the following amounts:''' | ||
| + | |||
| + | {| border="1" | ||
| + | |- | ||
| + | |Insert [20ng/μl] | ||
| + | |5μl | ||
| + | |- | ||
| + | |Vector | ||
| + | |1μl | ||
| + | |- | ||
| + | |Reaction buffer | ||
| + | |1μl | ||
| + | |- | ||
| + | |T4 DNA Ligase | ||
| + | |1μl | ||
| + | |- | ||
| + | |H2O | ||
| + | |2μl | ||
| + | |- | ||
| + | |Total | ||
| + | |10μl | ||
| + | |} | ||
| + | |||
| + | |||
| + | '''We used the follow denomination.''' | ||
| + | *'''Band purified vector : Pu''' | ||
| + | *'''iGEM's vector (Vial with green cover): P''' | ||
| + | *'''Digested vector: S''' | ||
| + | |||
| + | '''After one hour ligation we transformed by heat shock''' | ||
| + | |||
| + | '''and kept overnight the plates at 37ºC''' | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==''Friday''== | ||
| + | |||
| + | '''Today we discussed what we going to do with the AFP (anti freeze protein),''' | ||
| + | |||
| + | '''We decided transform by heat shock and plating it on kanamicina medium, then prepare''' | ||
| - | ''' | + | '''miniprep plasmid and cut it with ''Xba''I and ''Pst''I. We have to ligate it to the backbone J13002''' |
| - | ''' | + | '''the backbone had to be cut with ''Spe''I and ''Pst''I.''' |
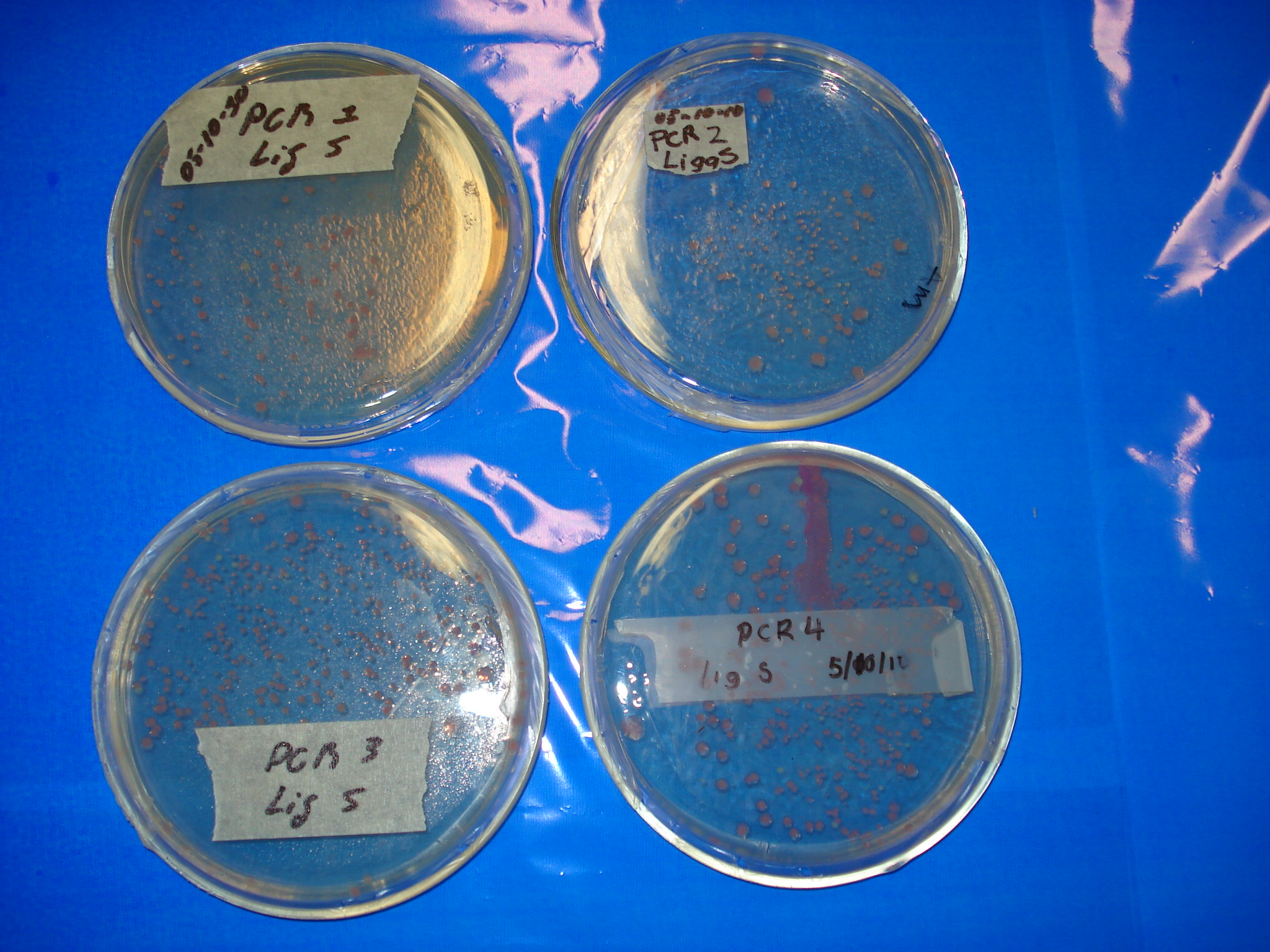
| - | ''' | + | '''Meanwhile, here are the previous day ligations.''' |
| - | ''' | + | *'''PCR1 Lig S Positive!!!!!!!''' |
| + | *'''PCR2 Lig S Positive!!!!''' | ||
| + | *'''PCR3 Lig S Positive!!!!''' | ||
| + | *'''PCR4 Lig Pu Positive!!!!''' | ||
| + | *'''PCR4 Lig S Positive!!!!''' | ||
| - | + | [[Image:Labo_254.JPG|400px]] | |
Latest revision as of 06:41, 27 October 2010
Week #4
27th September - 01 October 2010
Monday
Well...we are stuck with the vector. This week we have to get it.
- We have achieved a nano spectrophotometer and readings are below:
| ng/μl | 260/280 | 260/230 | |
| PsB1C3 | 0.60 | -0.73 | -0.0 |
| PCR 1 red | 427.70 | 1.71 | 1.71 |
| PCR 2 red | 483.20 | 1.74 | 1.71 |
| PCR 3 red | 410.70 | 1.76 | 2.02 |
| PCR 4 red | 431.05 | 1.61 | 1.88 |
| PCR 1 blue | 533.35 | 1.71 | 1.75 |
| PCR 2 blue | 536.00 | 1.72 | 1.82 |
| PCR 3 blue | 577.50 | 1.69 | 1.59 |
| PCR 4 blue | 627.55 | 1.70 | 1.56 |
| PsB1C3 | 4.10 | 2.62 | 1.01 |
- Yep, our trouble is the miniprep method
we have a low concentration of vector plasmid.
We have to work an try to get more plasmid and used diluted PCR products
is not possible achieve ligations with this vector an insert ratio.
Our advisor proposed use low Melting agarose
to extract the plasmid from the gel.
Tuesday
We ran with a low meelting agarose and cut off the band
we precipitated using alchol and salts in order
to get enough vector to do the ligations.
Wednesday
Plan
- We set up all to do the ligations.
- We digested both vector and insert with EcoRI and PstI .
- We ran a low melting agarose gel 1 hour at 80 volts, then cut off the band from gel.
At Claudia's lab we tried three two different kits to recover DNA from gel.
- Mobio ultra clean Microbial DNA kit
- Axygen.....
The digestions were done with following amounts.
| DNA | 20μl |
| Buffer NB2 | 3μl |
| EcoRI | 2μl |
| PstI | 2μl |
| H2O | 2.4μl |
| BSA | 0.6μl |
| Total | 30μl |
Thursday
Plan
We then proceed to diluted PCR fragments
For this we did the following operations:
Poner las fórmulas usadas en un formato bonito
We did the ligations using the following amounts:
| Insert [20ng/μl] | 5μl |
| Vector | 1μl |
| Reaction buffer | 1μl |
| T4 DNA Ligase | 1μl |
| H2O | 2μl |
| Total | 10μl |
We used the follow denomination.
- Band purified vector : Pu
- iGEM's vector (Vial with green cover): P
- Digested vector: S
After one hour ligation we transformed by heat shock
and kept overnight the plates at 37ºC
Friday
Today we discussed what we going to do with the AFP (anti freeze protein),
We decided transform by heat shock and plating it on kanamicina medium, then prepare
miniprep plasmid and cut it with XbaI and PstI. We have to ligate it to the backbone J13002
the backbone had to be cut with SpeI and PstI.
Meanwhile, here are the previous day ligations.
- PCR1 Lig S Positive!!!!!!!
- PCR2 Lig S Positive!!!!
- PCR3 Lig S Positive!!!!
- PCR4 Lig Pu Positive!!!!
- PCR4 Lig S Positive!!!!
 "
"