Team:Michigan/Virus Surface Display
From 2010.igem.org
(→In the Lab) |
|||
| (27 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Michigan Header}} | {{Michigan Header}} | ||
| + | {|cellspacing=0 style="background: transparent" | ||
| + | |-valign="top" | ||
| + | |width="700px" | | ||
| - | 7/10/2010 | + | '''7/10/2010''' |
13:00 | 13:00 | ||
| Line 8: | Line 11: | ||
| - | 7/13/2010 | + | '''7/13/2010''' |
9:00 | 9:00 | ||
| Line 16: | Line 19: | ||
| - | 7/15/2010 | + | '''7/15/2010''' |
4:30 | 4:30 | ||
| Line 22: | Line 25: | ||
| - | 7/18/2010 | + | '''7/18/2010''' |
5:00 | 5:00 | ||
| Line 28: | Line 31: | ||
| - | 7/21/2010 | + | '''7/21/2010''' |
6:00 | 6:00 | ||
| Line 34: | Line 37: | ||
| - | 7/23/2010 | + | '''7/23/2010''' |
9:00 | 9:00 | ||
| Line 61: | Line 64: | ||
| - | 7/25/2010 | + | '''7/25/2010''' |
5:00 | 5:00 | ||
• aliquot INP samples and primers for sequencing | • aliquot INP samples and primers for sequencing | ||
| + | |||
| + | |||
| + | '''7/26/2010''' | ||
| + | |||
| + | • Electroporation transformation of the following Biobrick parts: | ||
| + | 1. pBad/araC (I0500) | ||
| + | 2. Linker (K157013) | ||
| + | 3. GFP (E0040) | ||
| + | |||
| + | |||
| + | '''7/27/2010''' | ||
| + | 9:00 | ||
| + | |||
| + | • miniprep | ||
| + | |||
| + | |||
| + | '''7/28/2010''' | ||
| + | 4:00 | ||
| + | |||
| + | • digest | ||
| + | |||
| + | • gel electrophoresis | ||
| + | |||
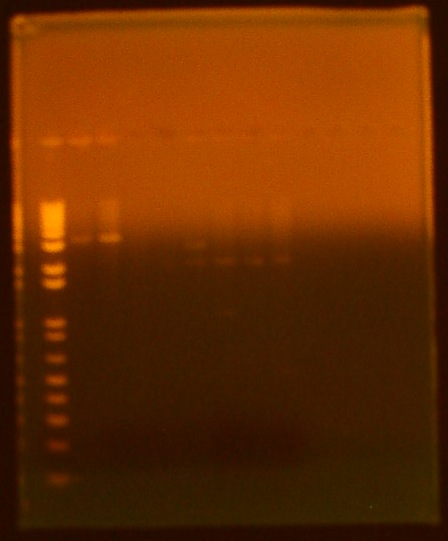
| + | [[Image:7-28biobrickgel.jpg|300px]] | ||
| + | |||
| + | Lane 1 (far right): Invitrogen 1 kb Plus ladder | ||
| + | |||
| + | Lane 2: GFP cut with XbaI and PstI | ||
| + | |||
| + | Lane 3: GFP cut with EcoRI and XbaI | ||
| + | |||
| + | Lane 4: uncut GFP plamid | ||
| + | |||
| + | Lane 5:INP cut with EcoRI and SpeI | ||
| + | |||
| + | Lane 6:INP cut with SpeI and PstI | ||
| + | |||
| + | Lane 7:uncut INP plasmid | ||
| + | |||
| + | Lane 8:Linker cut with XbaI and SpeI | ||
| + | |||
| + | Lane 9:Linker cut with SpeI and PstI | ||
| + | |||
| + | Lane 10: uncut Linker plasmid | ||
| + | |||
| + | Lane 11: OmpA cut with EcoRI and SpeI | ||
| + | |||
| + | Lane 12: OmpA cut with SpeI and PstI | ||
| + | |||
| + | Lane 13:uncut OmpA plasmid | ||
| + | |||
| + | |||
| + | '''7/30/2010''' | ||
| + | 10:00 | ||
| + | |||
| + | • Ligation of digested Biobrick parts: | ||
| + | - INP + Linker | ||
| + | - OmpA + GFP | ||
| + | - INP + GFP | ||
| + | • Heat shock transformation of ligation | ||
| + | |||
| + | |||
| + | '''8/2/2010''' | ||
| + | • Miniprep of INP-linker | ||
| + | • Nanodrop results: | ||
| + | INP + Linker construct ng/uL OD260/280 OD260/230 | ||
| + | 1.1 20.7 1.88 1.73 | ||
| + | 1.2 61.4 2.04 2.09 | ||
| + | 2.1 59.8 1.99 2.06 | ||
| + | 2.2 69.8 2.02 2.11 | ||
| + | |||
| + | '''8/3/2010''' | ||
| + | |||
| + | • EcoRI+SpeI and SpeI+PstI digests of INP + linker minipreps | ||
| + | |||
| + | • Gel electrophoresis results: | ||
| + | |||
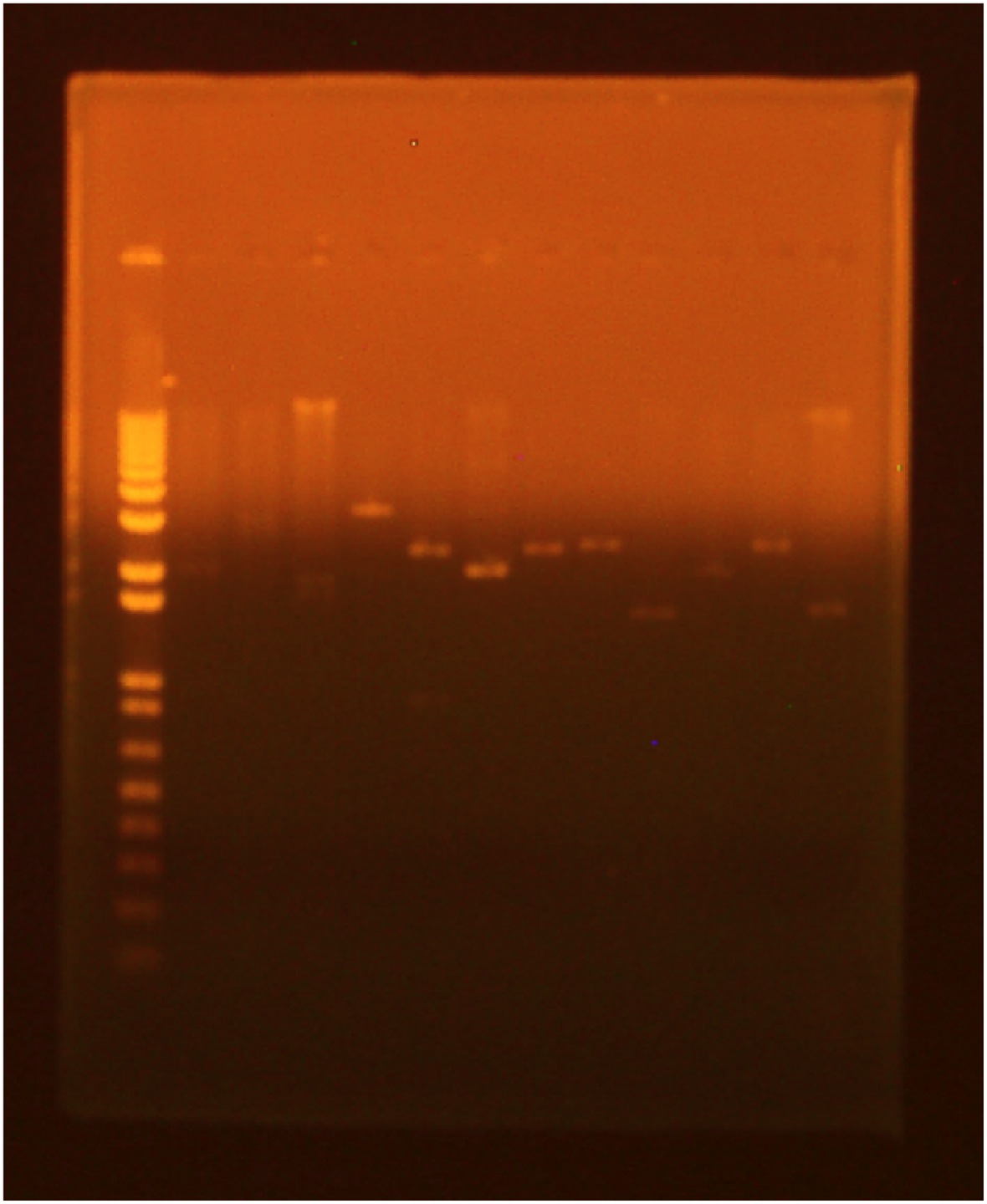
| + | [[Image:080310gel.jpg|250px]] | ||
| + | |||
| + | Lane 1 - 1 kb Plus Ladder | ||
| + | |||
| + | Lane 2 - INP + Linker 1.1 (EcoRI+SpeI) | ||
| + | |||
| + | Lane 3 - INP + Linker 1.2 (EcoRI+SpeI) | ||
| + | |||
| + | Lane 4 - INP + Linker 2.1 (EcoRI+SpeI) | ||
| + | |||
| + | Lane 5 - INP + Linker 2.2 (EcoRI+SpeI) | ||
| + | |||
| + | Lane 6 - INP + Linker 1.1 (SpeI+PstI) | ||
| + | |||
| + | Lane 7 - INP + Linker 1.2 (SpeI+PstI) | ||
| + | |||
| + | Lane 8 - INP + Linker 2.1 (SpeI+PstI) | ||
| + | |||
| + | Lane 9 - INP + Linker 2.2 (SpeI+PstI) | ||
| + | |||
| + | Lane 10 - INP 1 (EcoRI+SpeI) | ||
| + | |||
| + | Lane 11 - INP 2 (SpeI+PstI) | ||
| + | |||
| + | |||
| + | '''8/5/2010''' | ||
| + | |||
| + | • Inoculated 6 ml DH5α for ligation and transformation. | ||
| + | |||
| + | |||
| + | '''8/6/2010''' | ||
| + | |||
| + | • OmpA and GFP Ligations done: | ||
| + | 1. OmpA1 (insert) + GFP2 (backbone) | ||
| + | 2. OmpA2 (backbone) + GFP1 (insert) | ||
| + | |||
| + | • Heat shock transformation | ||
| + | |||
| + | |||
| + | '''8/9/2010''' | ||
| + | |||
| + | • Miniprep of 8/6 transformation, in addition to pBAD promoter and linker. | ||
| + | • Nanodrop results: | ||
| + | |||
| + | Sample OD260/280 OD260/230 ng/uL | ||
| + | 1. pBAD 1.72 8.91 11.5 | ||
| + | 2. OmpA+GFP 1 2.88 -3.52 4.5 | ||
| + | 3. OmpA+GFP 2 1.62 3.03 7.9 | ||
| + | 4. Linker 2.10 -12.00 9.4 | ||
| + | |||
| + | • 20 uL digest without water | ||
| + | - 5 uL NEB2 buffer | ||
| + | - 0.5 uL 100X BSA | ||
| + | - 1 uL of each enzyme | ||
| + | - 12.5 uL of DNA | ||
| + | |||
| + | • Digests: | ||
| + | 1. Linker 1 (EcoRI+SpeI) | ||
| + | 2. Linker 2 (EcoRI+SpeI) | ||
| + | 3. OmpA+GFP 1 (XbaI+PstI) | ||
| + | 4. OmpA+GFP 2 (XbaI+PstI) | ||
| + | 5. pBAD (SpeI+PstI) | ||
| + | |||
| + | |||
| + | '''8/10/10''' | ||
| + | |||
| + | • Gel of 8/9 digest results | ||
| + | |||
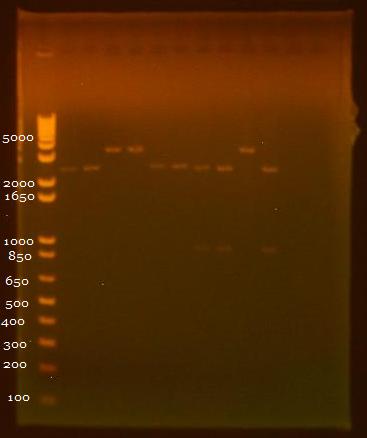
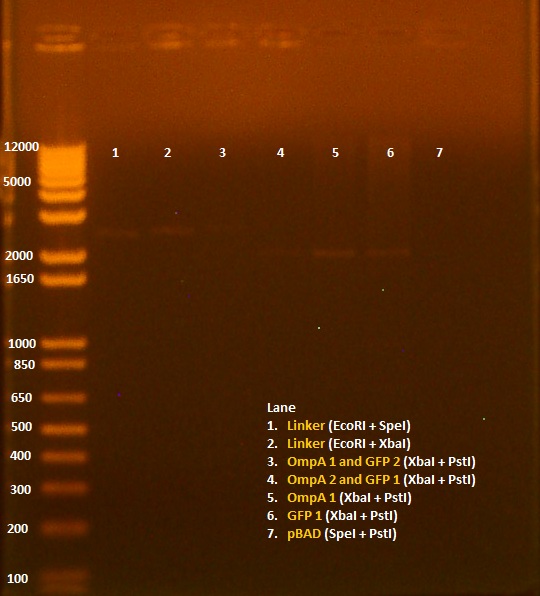
| + | [[Image:081010gel.jpg|350px]] | ||
| + | |||
| + | |||
| + | '''8/12/2010''' | ||
| + | |||
| + | • Due to poor digest results, we repeated the miniprep from 8/9, with additional colonies of OmpA+GFP picked. Also miniprepped are 2 expression plasmids (Backbone and Promoter) transformed earlier by another group. | ||
| + | |||
| + | Sample | ||
| + | |||
| + | |||
| + | |||
| + | |width="250px" style="background: transparent"| | ||
| + | |||
| + | ==='''In the Lab'''=== | ||
| + | |||
| + | [[Image:Virus01.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus02.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus03.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus04.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus05.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus06.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus07.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus08.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus09.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus10.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus11.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus12.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus13.jpg|middle|250px]] | ||
| + | |||
| + | [[Image:Virus14.jpg|middle|250px]] | ||
| + | |||
| + | |||
| + | |} | ||
Latest revision as of 04:44, 27 October 2010
|
7/10/2010 13:00 • digest I719015 (T7 GFPmut3b) and I719005(T7 Promoter) with EcoR1 and Pst1.
• inoculate competent cell culture • harvest I719015, K145001, K117008, K117002, K103006 from registry
• streak plate INP NC sample
• miniprep INP NC sample
• digest INP NC with EcoRI and SpeI
• gel electrophoresis of all biobricks lane 1 - ladder lane 2 - INP NC sample 1 (K265008) lane 3 - INP NC sample 2 (K265008) lane 4 - T7 GFPmut3b (I719015) lane 5 - T7 Promoter (I719005) lane 6 - T7 RNA polymerase (K145001) lane 7 - pLsrA-YFP (K117008) lane 8 - LsrA promoter (K117002) lane 9 - OmpA (K103006)
• aliquot INP samples and primers for sequencing
• Electroporation transformation of the following Biobrick parts: 1. pBad/araC (I0500) 2. Linker (K157013) 3. GFP (E0040)
• miniprep
• digest • gel electrophoresis Lane 1 (far right): Invitrogen 1 kb Plus ladder Lane 2: GFP cut with XbaI and PstI Lane 3: GFP cut with EcoRI and XbaI Lane 4: uncut GFP plamid Lane 5:INP cut with EcoRI and SpeI Lane 6:INP cut with SpeI and PstI Lane 7:uncut INP plasmid Lane 8:Linker cut with XbaI and SpeI Lane 9:Linker cut with SpeI and PstI Lane 10: uncut Linker plasmid Lane 11: OmpA cut with EcoRI and SpeI Lane 12: OmpA cut with SpeI and PstI Lane 13:uncut OmpA plasmid
• Ligation of digested Biobrick parts: - INP + Linker - OmpA + GFP - INP + GFP • Heat shock transformation of ligation
INP + Linker construct ng/uL OD260/280 OD260/230
1.1 20.7 1.88 1.73
1.2 61.4 2.04 2.09
2.1 59.8 1.99 2.06
2.2 69.8 2.02 2.11
8/3/2010 • EcoRI+SpeI and SpeI+PstI digests of INP + linker minipreps • Gel electrophoresis results: Lane 1 - 1 kb Plus Ladder Lane 2 - INP + Linker 1.1 (EcoRI+SpeI) Lane 3 - INP + Linker 1.2 (EcoRI+SpeI) Lane 4 - INP + Linker 2.1 (EcoRI+SpeI) Lane 5 - INP + Linker 2.2 (EcoRI+SpeI) Lane 6 - INP + Linker 1.1 (SpeI+PstI) Lane 7 - INP + Linker 1.2 (SpeI+PstI) Lane 8 - INP + Linker 2.1 (SpeI+PstI) Lane 9 - INP + Linker 2.2 (SpeI+PstI) Lane 10 - INP 1 (EcoRI+SpeI) Lane 11 - INP 2 (SpeI+PstI)
• Inoculated 6 ml DH5α for ligation and transformation.
• OmpA and GFP Ligations done: 1. OmpA1 (insert) + GFP2 (backbone) 2. OmpA2 (backbone) + GFP1 (insert) • Heat shock transformation
• Miniprep of 8/6 transformation, in addition to pBAD promoter and linker. • Nanodrop results: Sample OD260/280 OD260/230 ng/uL 1. pBAD 1.72 8.91 11.5 2. OmpA+GFP 1 2.88 -3.52 4.5 3. OmpA+GFP 2 1.62 3.03 7.9 4. Linker 2.10 -12.00 9.4 • 20 uL digest without water - 5 uL NEB2 buffer - 0.5 uL 100X BSA - 1 uL of each enzyme - 12.5 uL of DNA • Digests: 1. Linker 1 (EcoRI+SpeI) 2. Linker 2 (EcoRI+SpeI) 3. OmpA+GFP 1 (XbaI+PstI) 4. OmpA+GFP 2 (XbaI+PstI) 5. pBAD (SpeI+PstI)
• Gel of 8/9 digest results
• Due to poor digest results, we repeated the miniprep from 8/9, with additional colonies of OmpA+GFP picked. Also miniprepped are 2 expression plasmids (Backbone and Promoter) transformed earlier by another group. Sample
|
In the Lab
|
 "
"