Team:Stockholm/Lab work/Protocols
From 2010.igem.org
| Line 4: | Line 4: | ||
{| | {| | ||
|[[image:SU_Protocols_Icon.gif|200px]] | |[[image:SU_Protocols_Icon.gif|200px]] | ||
| - | |width=" | + | |width="10px"| |
| - | | | + | |width="590px"| |
| - | ==Competent cells (Nina & Johan)== | + | ==Protocols== |
| + | ===Competent cells (Nina & Johan)=== | ||
''From Morten Nørholm at the Department of Biochemistry & Biophysics Stockholm University'' | ''From Morten Nørholm at the Department of Biochemistry & Biophysics Stockholm University'' | ||
| Line 21: | Line 22: | ||
# Snapfreeze 100 mikroL aliquots in ice-cold Epps (in pre-chilled blocks). Store in -80 degree C freezer. | # Snapfreeze 100 mikroL aliquots in ice-cold Epps (in pre-chilled blocks). Store in -80 degree C freezer. | ||
| - | ==Competent cells (Andreas & Mimmi)== | + | ===Competent cells (Andreas & Mimmi)=== |
''Based and modified from the [http://openwetware.org/wiki/TOP10_chemically_competent_cells Top10 protocol by the Knight lab]'' | ''Based and modified from the [http://openwetware.org/wiki/TOP10_chemically_competent_cells Top10 protocol by the Knight lab]'' | ||
| Line 40: | Line 41: | ||
# Aliquot 100 μl samples of competent cells into 1.5 ml vials and store in -80°C, or transform immediately. | # Aliquot 100 μl samples of competent cells into 1.5 ml vials and store in -80°C, or transform immediately. | ||
| - | ==Transformation (Nina & Johan)== | + | ===Transformation (Nina & Johan)=== |
''From NEB 5-alpha competent E.coli (High Efficiency) NEW ENGLAND BioLabs'' | ''From NEB 5-alpha competent E.coli (High Efficiency) NEW ENGLAND BioLabs'' | ||
| Line 54: | Line 55: | ||
# Spread 50-100 μl of each sample onto a selection plate and incubate overnight at 37°C. | # Spread 50-100 μl of each sample onto a selection plate and incubate overnight at 37°C. | ||
| - | ==Transformation (Andreas & Mimmi)== | + | ===Transformation (Andreas & Mimmi)=== |
# Add 1 μl plasmid to 100 μl thawed, competent cells of choice. Hold cells on ice for 30 min. | # Add 1 μl plasmid to 100 μl thawed, competent cells of choice. Hold cells on ice for 30 min. | ||
# Heat-shock cells for 55 sec in 42 °C. Return to ice. | # Heat-shock cells for 55 sec in 42 °C. Return to ice. | ||
| Line 64: | Line 65: | ||
# Incubate in 37 °C ON. | # Incubate in 37 °C ON. | ||
| - | ==Quick transformation (Andreas & Mimmi)== | + | ===Quick transformation (Andreas & Mimmi)=== |
# Add 1-3 μl plasmid to 100 μl thawed, competent Top10 cells. Hold cells on ice for 5 min. | # Add 1-3 μl plasmid to 100 μl thawed, competent Top10 cells. Hold cells on ice for 5 min. | ||
# Heat-shock cells for 30 sec in 42 °C. Return to ice. | # Heat-shock cells for 30 sec in 42 °C. Return to ice. | ||
| Line 70: | Line 71: | ||
# Incubate in 37 °C ON. | # Incubate in 37 °C ON. | ||
| - | ==Colony PCR verification (Andreas & Mimmi)== | + | ===Colony PCR verification (Andreas & Mimmi)=== |
# Pick four colonies and resuspend each in 10 μl LB. | # Pick four colonies and resuspend each in 10 μl LB. | ||
#* Let incubate in RT while preparing PCR tubes. | #* Let incubate in RT while preparing PCR tubes. | ||
| Line 87: | Line 88: | ||
# Analyze PCR products by agarose gel electrophoresis. | # Analyze PCR products by agarose gel electrophoresis. | ||
| - | ==Site-directed mutagenesis== | + | ===Site-directed mutagenesis=== |
''Based on the QuikChange® Site-Directed Mutagenesis Kit'' | ''Based on the QuikChange® Site-Directed Mutagenesis Kit'' | ||
* Design two complimentary oligonucleotides containing the desired mutation with https://www.genomics.agilent.com/CollectionSubpage.aspx?PageType=Tool&SubPageType=ToolQCPD&PageID=15 | * Design two complimentary oligonucleotides containing the desired mutation with https://www.genomics.agilent.com/CollectionSubpage.aspx?PageType=Tool&SubPageType=ToolQCPD&PageID=15 | ||
| Line 111: | Line 112: | ||
---- | ---- | ||
| - | ==SDS-PAGE mixtures (Nina & Johan)== | + | ===SDS-PAGE mixtures (Nina & Johan)=== |
''From Robert Daniels at the Department of Biochemistry & Biophysics Stockholm University'' | ''From Robert Daniels at the Department of Biochemistry & Biophysics Stockholm University'' | ||
| Line 117: | Line 118: | ||
---- | ---- | ||
| - | ==Mini prep(Nina & Johan)== | + | ===Mini prep (Nina & Johan)=== |
''Based on the QIAprep Spin Miniprep Kit Using a Microcentrifuge'' | ''Based on the QIAprep Spin Miniprep Kit Using a Microcentrifuge'' | ||
| Line 141: | Line 142: | ||
---- | ---- | ||
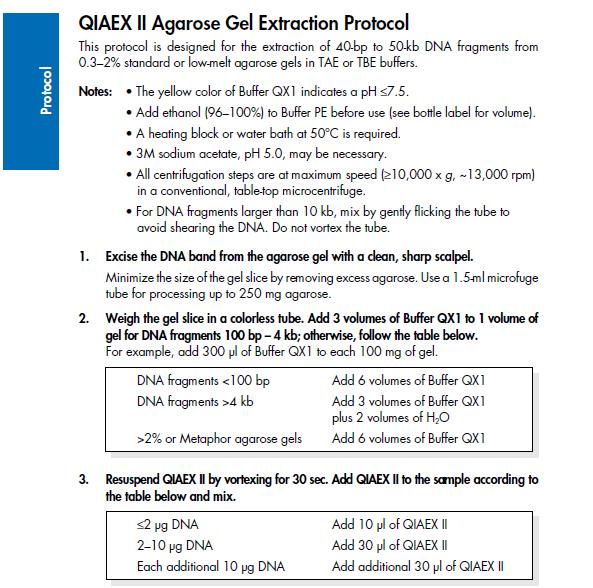
| - | ==Agarose gel clean up (Nina & Johan)== | + | ===Agarose gel clean up (Nina & Johan)=== |
''Based on the QIAprep II Handbook'' | ''Based on the QIAprep II Handbook'' | ||
| Line 153: | Line 154: | ||
[[Image:Protocol3.jpg]] | [[Image:Protocol3.jpg]] | ||
| - | ==IgG protease activity assay== | + | ===IgG protease activity assay=== |
Based on an experiment by [http://www.ncbi.nlm.nih.gov/pubmed/20441890 Abuknesha, ''et al'' (2010)]. | Based on an experiment by [http://www.ncbi.nlm.nih.gov/pubmed/20441890 Abuknesha, ''et al'' (2010)]. | ||
| - | ===Materials=== | + | ====Materials==== |
*10 ml culture tubes | *10 ml culture tubes | ||
| Line 173: | Line 174: | ||
*SureBlue™ TMB Microwell Peroxidase Substrate | *SureBlue™ TMB Microwell Peroxidase Substrate | ||
| - | ===Procedures=== | + | ====Procedures==== |
| - | ====Day 0==== | + | =====Day 0===== |
#Set ON cultures in 5 ml LB + 100 μg/ml Amp, 37 °C, 225 rpm | #Set ON cultures in 5 ml LB + 100 μg/ml Amp, 37 °C, 225 rpm | ||
#*BL21 IgGp | #*BL21 IgGp | ||
#*BL21 SOD (or other negative control) | #*BL21 SOD (or other negative control) | ||
| - | ====Day 1==== | + | =====Day 1===== |
#Inoculate 200 μl of each ON culture (x2 for IgGp) into 20 ml fresh LB with 100 μg/ml Amp. Grow at 37 °C, 225 rpm until an OD<sub>600</sub> of ≈0.5. | #Inoculate 200 μl of each ON culture (x2 for IgGp) into 20 ml fresh LB with 100 μg/ml Amp. Grow at 37 °C, 225 rpm until an OD<sub>600</sub> of ≈0.5. | ||
#Induce protein expression by adding IPTG to a final concentration of 0.3 mM (e.g. 60 μl 0.1 M IPTG) to one of each culture type. Leave the second IgGp culture uninduced (negative control). Continue incubation for two hours. | #Induce protein expression by adding IPTG to a final concentration of 0.3 mM (e.g. 60 μl 0.1 M IPTG) to one of each culture type. Leave the second IgGp culture uninduced (negative control). Continue incubation for two hours. | ||
| Line 201: | Line 202: | ||
#Add 100 μl of the protease extracts to the corresponding tubes (12 tubes). Add 100 μl PBS to the remaining two negative control tubes. Incubate at 37 °C ON (≈16 h) with tilt mixing. | #Add 100 μl of the protease extracts to the corresponding tubes (12 tubes). Add 100 μl PBS to the remaining two negative control tubes. Incubate at 37 °C ON (≈16 h) with tilt mixing. | ||
| - | ====Day 2==== | + | =====Day 2===== |
#Spin down the IgG-Agarose at 13,000 x g, 3 min. Transfer 80 μl of each supernatant (no pellet!) to new Eppendorf tubes. | #Spin down the IgG-Agarose at 13,000 x g, 3 min. Transfer 80 μl of each supernatant (no pellet!) to new Eppendorf tubes. | ||
#Add 100 μl Sure Blue™ peroxidase substrate solution. Incubate in room temperature and tap gently until a blue color develops (≈30 min). | #Add 100 μl Sure Blue™ peroxidase substrate solution. Incubate in room temperature and tap gently until a blue color develops (≈30 min). | ||
Revision as of 19:29, 26 October 2010
ProtocolsCompetent cells (Nina & Johan)From Morten Nørholm at the Department of Biochemistry & Biophysics Stockholm University
Competent cells (Andreas & Mimmi)Based and modified from the [http://openwetware.org/wiki/TOP10_chemically_competent_cells Top10 protocol by the Knight lab] MaterialsCCMB80 buffer Procedures
Transformation (Nina & Johan)From NEB 5-alpha competent E.coli (High Efficiency) NEW ENGLAND BioLabs
Transformation (Andreas & Mimmi)
Quick transformation (Andreas & Mimmi)
Colony PCR verification (Andreas & Mimmi)
Site-directed mutagenesisBased on the QuikChange® Site-Directed Mutagenesis Kit
SDS-PAGE mixtures (Nina & Johan)From Robert Daniels at the Department of Biochemistry & Biophysics Stockholm University
Mini prep (Nina & Johan)Based on the QIAprep Spin Miniprep Kit Using a Microcentrifuge 1. Centrifuge sample in 4 °C, 10 min, 4000 rpm and resuspend pelleted bacterial cells with Buffer P1 (250 ul/5ml bacterial sample) and transfer to a microcentrifuge tube. 2. Add 250 ul buffer P2, invert the tubes 4-6 times. 3. Add 350 ul buffer N3, invert the tubes 4-6 times with powerful strokes. 4. Centrifuge 10 min, 13000 rpm in a table-top microcentrifuge. 5. Transfer the supernatant to the QIAprep spin column. 6. Centrifuge for 1 min, 13000 rpm. Discard the flow-through. 7. Add 0.5 ml buffer PB and centrifuge for 1 min, 13000 rpm. Discard the flow-through. 8. Add 0.75 ml buffer PE and centrifuge for 1 min, 13000 rpm. 9. Discard the flow-through and centrifuge again the same way. 10. Place the column in a clean 1.5 ml microcentrifuge tube. Add 35 ul water to the center of the QIAprep spin column, let stand for 1 min, and centrifuge for 1 min, 13000 rpm. Agarose gel clean up (Nina & Johan)Based on the QIAprep II Handbook IgG protease activity assayBased on an experiment by [http://www.ncbi.nlm.nih.gov/pubmed/20441890 Abuknesha, et al (2010)]. Materials
ProceduresDay 0
Day 1
Day 2
|
 |

|
 |

|
 |

|

|

|
 "
"