Team:Stockholm/6 September 2010
From 2010.igem.org
(→Andreas) |
m |
||
| (One intermediate revision not shown) | |||
| Line 33: | Line 33: | ||
|} | |} | ||
| - | |||
==Andreas== | ==Andreas== | ||
| Line 223: | Line 222: | ||
Tubes stored in -20 °C. | Tubes stored in -20 °C. | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Latest revision as of 11:00, 26 October 2010
Contents |
Mimmi
MITF-M
Gel
| well | sample |
|---|---|
| 1 | 1kb ladder |
| 2 | MITF-M 1 |
| 3 | MITF-M 2 |
| 4 | MITF-M 3 |
| 5 | MITF-M 4 |
| 6 | positive control |
| 7 | blank |
Andreas
Cloning of N-CPPs into pSB1C3
Transformation results
From 3/9
More growth on the 2 μl plate than on the 5 μl plate, indicating what I already suspected: contamination in transformation. Discarded both plates and restarted cloning.
Digestion
[N-CPP plasmid] = 672 ng/μl
| N-CPP | |
|---|---|
| 10X buffer Tango | 2 |
| DNA (1 μg) | 1.5 |
| dH2O | 12.5 |
| XbaI | 1 |
| AgeI | 4 |
| 20 μl |
- Incubation: 37 °C, 3 h
- Inactivation: 80 °C, 20 min
Control digestions
Did two control digestions to analyze the N-CPP plasmid vector size.
| A+S | ApaI | |
|---|---|---|
| 10X buffer Tango | 2 | 2 |
| DNA | 1 | 1 |
| dH2O | 15 | 16 |
| FD ApaI | 1 | 1 |
| FD SmaI | 1 | 0 |
| 20 μl | 20 μl |
- Incubation: 37 °C, 30 min
- Inactivation: 65 °C, 5 min
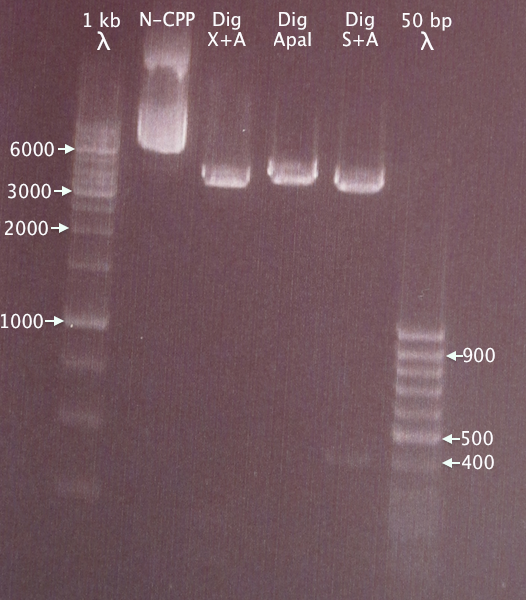
Gel verification
1 % agarose, 120 V, 40 min
- N-CPP: undigested N-CPP plasmid
- Dig X+A: N-CPP plasmid digested with XbaI & AgeI
- Dig ApaI: Linearized N-CPP plasmid digested with ApaI
- Dig S+A: N-CPP plasmid digested with SmaI and ApaI, excising the 391 bp N-CPP cluster.
Results
Linearized plasmid seems to be ≈5 kb long. Undigested plasmid moved slower; this was probably the result of the lane being too crowded.
Ligation
Vector: Dig pSB1C3 X+A EXTR 1
| 1 | 2* | |
|---|---|---|
| 5X Rapid Ligation buffer | 4 | 4 |
| Vector DNA | 4 | 4 |
| Insert DNA | 11 | 9 |
| dH2O | 0 | 2 |
| T4 DNA ligase | 1 | 1 |
| 22 μl † | 20 μl |
† Accidentally added 2 μl FastAP alkaline phosphatase to ligation mix 1. Enzyme inactivated at 75 °C, 10 min, prior to ligation.
- Incubation: 22 °C, 10 min
Transformation
Performed by Mimmi
Standard transformation, except:
- 30 min incubation in 37 °C
- 3 μl ligation mix
Cells grown on Cm 25 plates.
PCR
Since we've experienced some problems with our N-CPP clonings, a PCR of each individual N-CPP was performed for individual cloning, in case the current cloning doesn't work.
| Reactions & primers | ||
|---|---|---|
| Forward | Reverse | |
| N-Tra10 | VF2 | VR |
| N-TAT | pEXf | pEXr |
| N-LMWP | pGexf | pGexr |
| N-CPP cluster | VF2 | pGexr |
| PCR tubes | |
|---|---|
| 10X Pfu buffer | 5 |
| dNTP, 10 mM | 1 |
| dH2O | 41 |
| Template DNA | 0.5 |
| Forw. primer | 1 |
| Rev. primer | 1 |
| Pfu DNA pol. | 0.5 |
| 50 μl | |
Standard colony PCR settings
- Elongation: 1:15
Tubes stored in -20 °C.
 |

|
 |

|
 |

|

|

|
 "
"