Team:Stockholm/22 July 2010
From 2010.igem.org
(Difference between revisions)
m (New page: {{Stockholm/Top2}} __TOC__ == Hassan == tyrp1 nalp1 mitf CGRP S100B foxd3 GGA1 LMP7 TAPBP) |
m |
||
| (3 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Hassan == | == Hassan == | ||
| - | tyrp1 | + | *tyrp1 |
| - | nalp1 | + | *nalp1 |
| - | mitf | + | *mitf |
| - | CGRP | + | *CGRP |
| - | S100B | + | *S100B |
| - | foxd3 | + | *foxd3 |
| - | GGA1 | + | *GGA1 |
| - | LMP7 | + | *LMP7 |
| - | TAPBP | + | *TAPBP |
| + | |||
| + | ==Johan== | ||
| + | |||
| + | ===PCR=== | ||
| + | |||
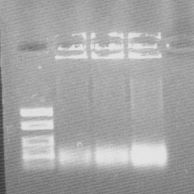
| + | The PCR samples that was run on a gel to verify that the colonies had been successfully site-directed mutagenesised seemed to be lost, and a new colony PCR was performed to verify the site-directed mutagenesis before cleaving and adding it to the target vectors. | ||
| + | |||
| + | [[Image:Agarose Johan bFGF 22july 22july.jpg]] | ||
| + | |||
| + | The ladder is FastRuler DNA ladder low range, but the bands does not show the expected size. Did I mistakenly use the FastRuler DNA ladder middle range? | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Mimmi == | ||
| + | |||
| + | === LMWP === | ||
| + | |||
| + | ==== glycerol stock + preparation of plasmids ==== | ||
| + | |||
| + | |||
| + | {| | ||
| + | ! Mix | ||
| + | | | ||
| + | |- | ||
| + | | LB | ||
| + | | 12ml | ||
| + | |- | ||
| + | | Amp | ||
| + | | 24µl | ||
| + | |- | ||
| + | | ON culture | ||
| + | | 120µl | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | *Shake in 37°C, ~200rpm, ~3h | ||
| + | |||
| + | *Add 1600µl culture to pre-made jar with 400µl sterile glycerol | ||
| + | |||
| + | *Take 5ml culture and follow the mini plasmid prep. kit 1 protocol. | ||
| + | |||
| + | **Wash 2X with EtOH solution | ||
| + | |||
| + | **Dilute in 50µl HPL6 H<sub>2</sub>O | ||
| + | |||
| + | :--> in the freezer (plasmid & DNA ) pEX.BBa_J18930A -> 32A | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==== ligation ==== | ||
| + | |||
| + | |||
| + | {| | ||
| + | | LMWP_N_F1 | ||
| + | | 2µg/µl | ||
| + | |- | ||
| + | | LMWP_CN_F2 | ||
| + | | 1.6µg/µl | ||
| + | |- | ||
| + | | LMWP_CN_R1 | ||
| + | | 2µg/µl | ||
| + | |- | ||
| + | | LMWP_N_R2 | ||
| + | | 1.5µg/µl | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | LMWP_C_F1 | ||
| + | | 1.8µg/µl | ||
| + | |- | ||
| + | | LMWP_C_R2 | ||
| + | | 1.5µg/µl | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | ! Mix | ||
| + | | (µl) | ||
| + | |- | ||
| + | | F1 | ||
| + | | 1 | ||
| + | |- | ||
| + | | F2 | ||
| + | | 1 | ||
| + | |- | ||
| + | | R1 | ||
| + | | 1 | ||
| + | |- | ||
| + | | R2 | ||
| + | | 1 | ||
| + | |- | ||
| + | | H<sub>2</sub>O | ||
| + | | 4 | ||
| + | |- | ||
| + | | DNA sol. | ||
| + | | 42 | ||
| + | |- | ||
| + | | align="right" | tot | ||
| + | | 50µl | ||
| + | |} | ||
| + | |||
| + | |||
| + | *Heatshock 90°C 3min | ||
| + | |||
| + | *Incubate 37°C 1h | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | ! Mix | ||
| + | | | ||
| + | | 3X | ||
| + | |- | ||
| + | | H<sub>2</sub>O | ||
| + | | 5 | ||
| + | | rowspan="6" | | ||
| + | |- | ||
| + | | plasmid | ||
| + | | 2 | ||
| + | |- | ||
| + | | T4 buffer | ||
| + | | 1 | ||
| + | |- | ||
| + | | T4 ligase | ||
| + | | 1 | ||
| + | |- | ||
| + | | DNA | ||
| + | | 1 | ||
| + | |- | ||
| + | | align="right" | tot | ||
| + | | 10µl | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | *Incubate 22°C 1-3h | ||
| + | |||
| + | *Thaw 3x100µl Top10 competent cells | ||
| + | |||
| + | *Add 1µl plasmid | ||
| + | |||
| + | *Hold on ice 30min | ||
| + | |||
| + | *Heat shock 42°C 50s | ||
| + | |||
| + | *Cool down on ice | ||
| + | |||
| + | *Add 900µl LB | ||
| + | :Incubate 37°C 1h 250rpm | ||
| + | |||
| + | *Spin down cells 13000rpm 15s | ||
| + | |||
| + | *Remove 900µl, resuspend in remaining 100µl | ||
| + | |||
| + | *Plate 100µl on LB agar plates with Cm<sup>r</sup> | ||
| + | |||
| + | *Grow in 37°C ON | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Latest revision as of 10:51, 26 October 2010
Contents |
Hassan
- tyrp1
- nalp1
- mitf
- CGRP
- S100B
- foxd3
- GGA1
- LMP7
- TAPBP
Johan
PCR
The PCR samples that was run on a gel to verify that the colonies had been successfully site-directed mutagenesised seemed to be lost, and a new colony PCR was performed to verify the site-directed mutagenesis before cleaving and adding it to the target vectors.
The ladder is FastRuler DNA ladder low range, but the bands does not show the expected size. Did I mistakenly use the FastRuler DNA ladder middle range?
Mimmi
LMWP
glycerol stock + preparation of plasmids
| Mix | |
|---|---|
| LB | 12ml |
| Amp | 24µl |
| ON culture | 120µl |
- Shake in 37°C, ~200rpm, ~3h
- Add 1600µl culture to pre-made jar with 400µl sterile glycerol
- Take 5ml culture and follow the mini plasmid prep. kit 1 protocol.
- Wash 2X with EtOH solution
- Dilute in 50µl HPL6 H2O
- --> in the freezer (plasmid & DNA ) pEX.BBa_J18930A -> 32A
ligation
| LMWP_N_F1 | 2µg/µl |
| LMWP_CN_F2 | 1.6µg/µl |
| LMWP_CN_R1 | 2µg/µl |
| LMWP_N_R2 | 1.5µg/µl |
| LMWP_C_F1 | 1.8µg/µl |
| LMWP_C_R2 | 1.5µg/µl |
| Mix | (µl) |
|---|---|
| F1 | 1 |
| F2 | 1 |
| R1 | 1 |
| R2 | 1 |
| H2O | 4 |
| DNA sol. | 42 |
| tot | 50µl |
- Heatshock 90°C 3min
- Incubate 37°C 1h
| Mix | 3X | |
|---|---|---|
| H2O | 5 | |
| plasmid | 2 | |
| T4 buffer | 1 | |
| T4 ligase | 1 | |
| DNA | 1 | |
| tot | 10µl |
- Incubate 22°C 1-3h
- Thaw 3x100µl Top10 competent cells
- Add 1µl plasmid
- Hold on ice 30min
- Heat shock 42°C 50s
- Cool down on ice
- Add 900µl LB
- Incubate 37°C 1h 250rpm
- Spin down cells 13000rpm 15s
- Remove 900µl, resuspend in remaining 100µl
- Plate 100µl on LB agar plates with Cmr
- Grow in 37°C ON
 |

|
 |

|
 |

|

|

|
 "
"