Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test16settembre
From 2010.igem.org
(→Methods) |
(→Methods) |
||
| Line 27: | Line 27: | ||
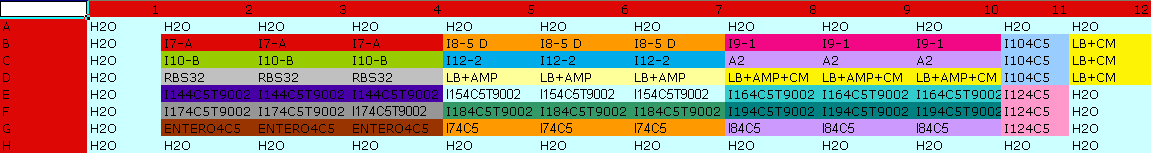
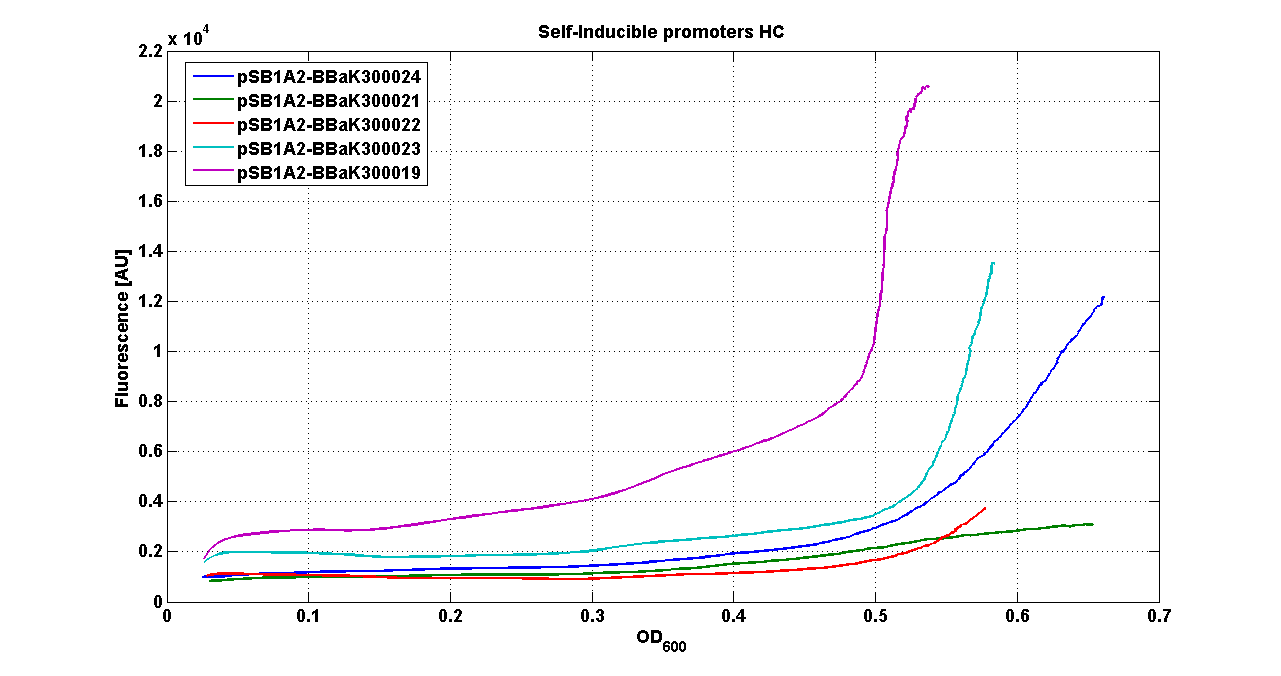
* <partinfo>pSB1A2</partinfo> - <partinfo>BBa_K300022</partinfo> (wiki name: I9) | * <partinfo>pSB1A2</partinfo> - <partinfo>BBa_K300022</partinfo> (wiki name: I9) | ||
* <partinfo>pSB1A2</partinfo> - <partinfo>BBa_K300023</partinfo> (wiki name: I10) | * <partinfo>pSB1A2</partinfo> - <partinfo>BBa_K300023</partinfo> (wiki name: I10) | ||
| - | * <partinfo>pSB1A2</partinfo> - <partinfo> | + | * <partinfo>pSB1A2</partinfo> - <partinfo>BBa_K300019</partinfo> (wiki name: I12) |
* <partinfo>pSB1A2</partinfo> - <partinfo>K173001</partinfo> (positive control) | * <partinfo>pSB1A2</partinfo> - <partinfo>K173001</partinfo> (positive control) | ||
* <partinfo>pSB1A2</partinfo> - <partinfo>BBa_B0032</partinfo> (negative control) | * <partinfo>pSB1A2</partinfo> - <partinfo>BBa_B0032</partinfo> (negative control) | ||
| Line 40: | Line 40: | ||
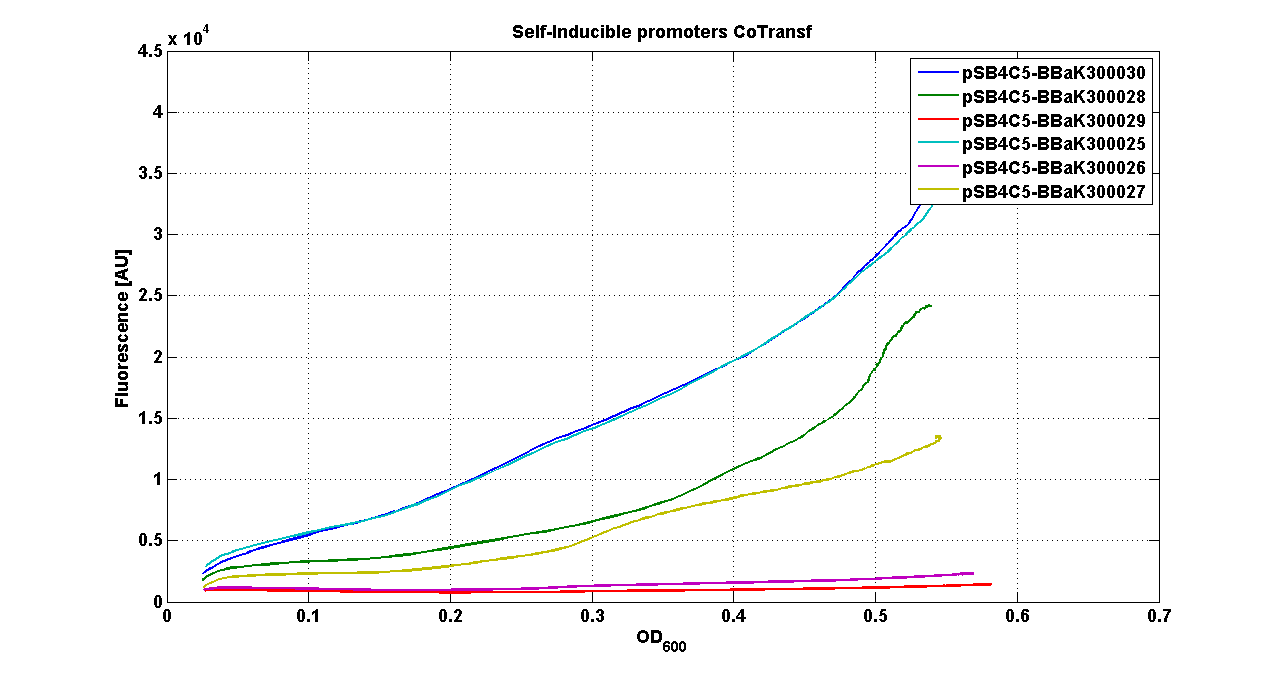
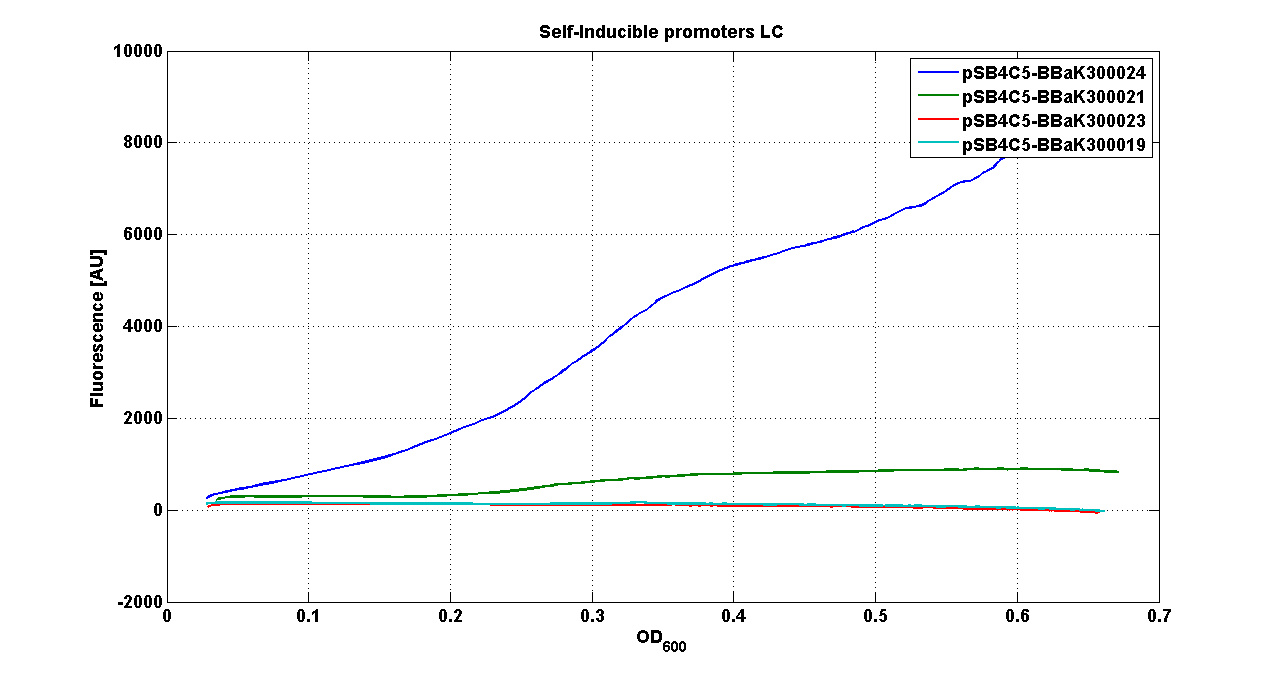
* <partinfo>pSB4C5</partinfo> - <partinfo>BBa_K300021</partinfo> (wiki name: I8-4C5) | * <partinfo>pSB4C5</partinfo> - <partinfo>BBa_K300021</partinfo> (wiki name: I8-4C5) | ||
* <partinfo>pSB4C5</partinfo> - <partinfo>BBa_K300023</partinfo> (wiki name: I10-4C5) | * <partinfo>pSB4C5</partinfo> - <partinfo>BBa_K300023</partinfo> (wiki name: I10-4C5) | ||
| - | * <partinfo>pSB4C5</partinfo> - <partinfo> | + | * <partinfo>pSB4C5</partinfo> - <partinfo>BBa_K300019</partinfo> (wiki name: I12-4C5) |
They were let grow ON at +37°C, 220 rpm. | They were let grow ON at +37°C, 220 rpm. | ||
Revision as of 21:25, 25 October 2010
|
|
||||||||||||||||
|
|
|
||||||||||||||
 "
"