Team:UNIPV-Pavia/Calendar/July/settimana2
From 2010.igem.org
(→July, 8th) |
m (→July, 6th) |
||
| (28 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
<td valign="top"> | <td valign="top"> | ||
<table border="0" align="center" width="100%"><tr><td align="justify" valign="top" style="padding:20px"> | <table border="0" align="center" width="100%"><tr><td align="justify" valign="top" style="padding:20px"> | ||
| - | |||
| + | <table class="menu" border="0" width="100%"> | ||
| + | <tr> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana1|Week 1]] | ||
| + | </td> | ||
| + | <td align="center" style="padding:0; height:20px"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana2|Week 2]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana3|Week 3]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana4|Week 4]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana5|Week 5]] | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | <html><p align="center"><font size="4"><b>JULY: WEEK 2</b></font></p></html><hr><br> | ||
| + | <html><a name="indice"/></html> | ||
==July, 5th== | ==July, 5th== | ||
| + | LB agar plates prepared: | ||
| + | *LB+Amp 100 (500ml) | ||
| + | *LB+Cm 34 (500ml) | ||
| + | *LB (250ml) | ||
| + | *LB+Cm 12.5 (250ml) | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 6th== | ==July, 6th== | ||
| + | We decided to perform a TECAN test to evaluate the strength of some promoters belonging to the Anderson Promoters Collection. Inoculum from glycerol stocks (8ul in 5ml LB) for: | ||
| + | *<partinfo>BBa_J23101</partinfo> | ||
| + | *<partinfo>BBa_J23110</partinfo> | ||
| + | *<partinfo>BBa_J23116</partinfo> | ||
| + | *<partinfo>BBa_J23118</partinfo> | ||
| + | *<partinfo>BBa_J23114</partinfo> | ||
| + | *<partinfo>BBa_B0033</partinfo> | ||
| + | |||
| + | TECAN experiment started at 10:20am. | ||
| + | |||
| + | In the morning one single colony was picked for BW53474 E. coli strain and glycerol stock was prepared after about 7 hours (37°C 220rpm) | ||
| + | |||
| + | Inoculum of: | ||
| + | *BW53474 (falcon containing culture for glycerol stock was re-filled with 5ml LB) | ||
| + | *MG1655 (from glycerol stock) | ||
| + | *BW25141 (from glycerol stock) | ||
| + | *BW25142 (from glycerol stock) | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 7th== | ==July, 7th== | ||
| + | Competent cells preparation for: | ||
| + | *MG1655 | ||
| + | *BW53474 | ||
| + | *BW25141 | ||
| + | *BW25142 | ||
| + | |||
| + | PCR was performed to amplify Phasins: | ||
| + | |||
| + | All parts were correct!! :) | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 8th== | ==July, 8th== | ||
| - | Transformation of | + | Transformation of 1ul (~4ng) of miniprepped <partinfo>BBa_J23118</partinfo> into 100ul of home-made competent cells |
*MG1655 | *MG1655 | ||
*BW25141 | *BW25141 | ||
*BW25142 | *BW25142 | ||
*BW53474 | *BW53474 | ||
| - | * | + | *DH5-alpha (as control) |
to verify the transformation efficiency. | to verify the transformation efficiency. | ||
| - | Transformed bacteria | + | Transformed bacteria were plated on LB+Amp and left overnight in oven, 37°C. |
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 9th== | ==July, 9th== | ||
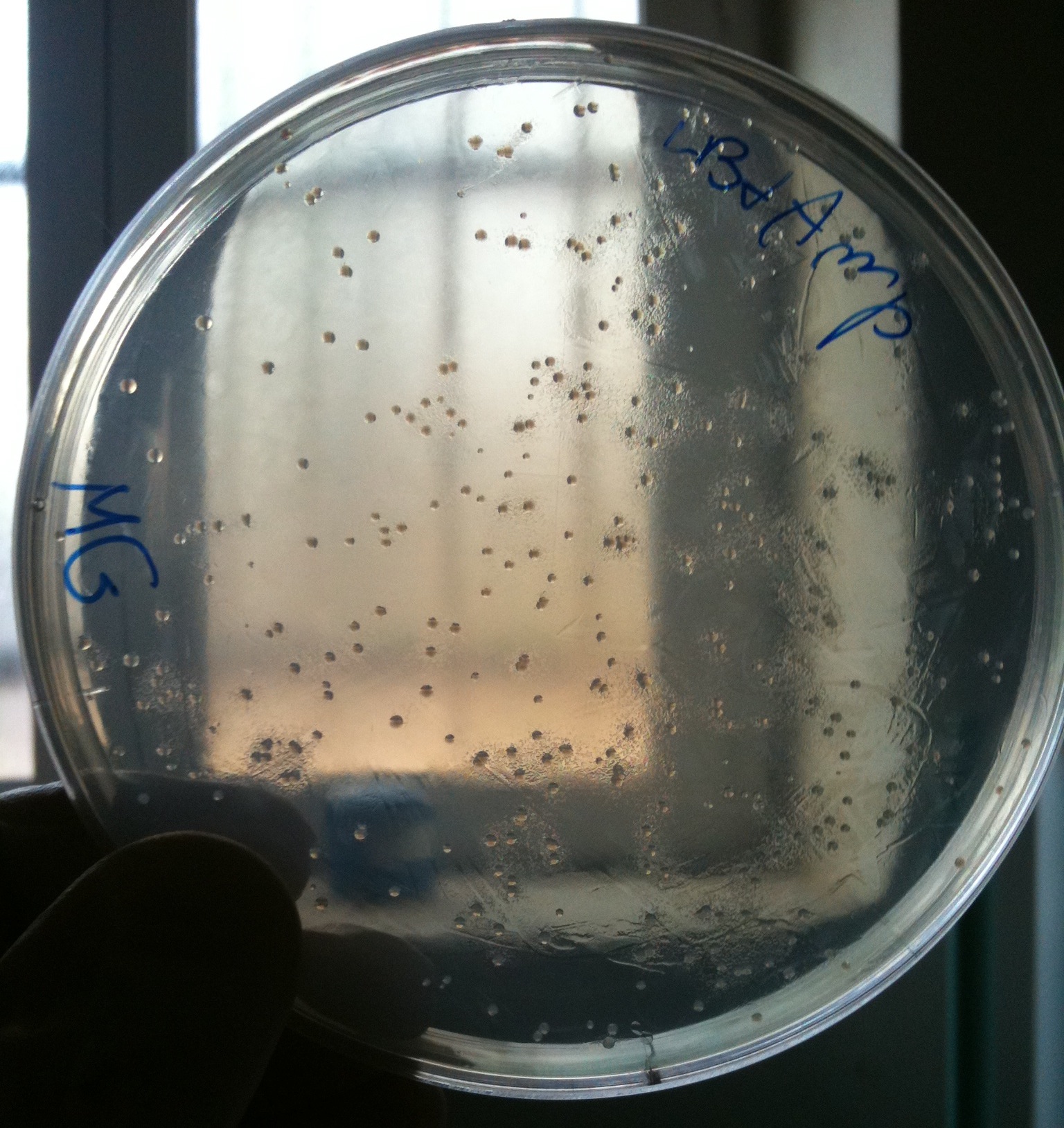
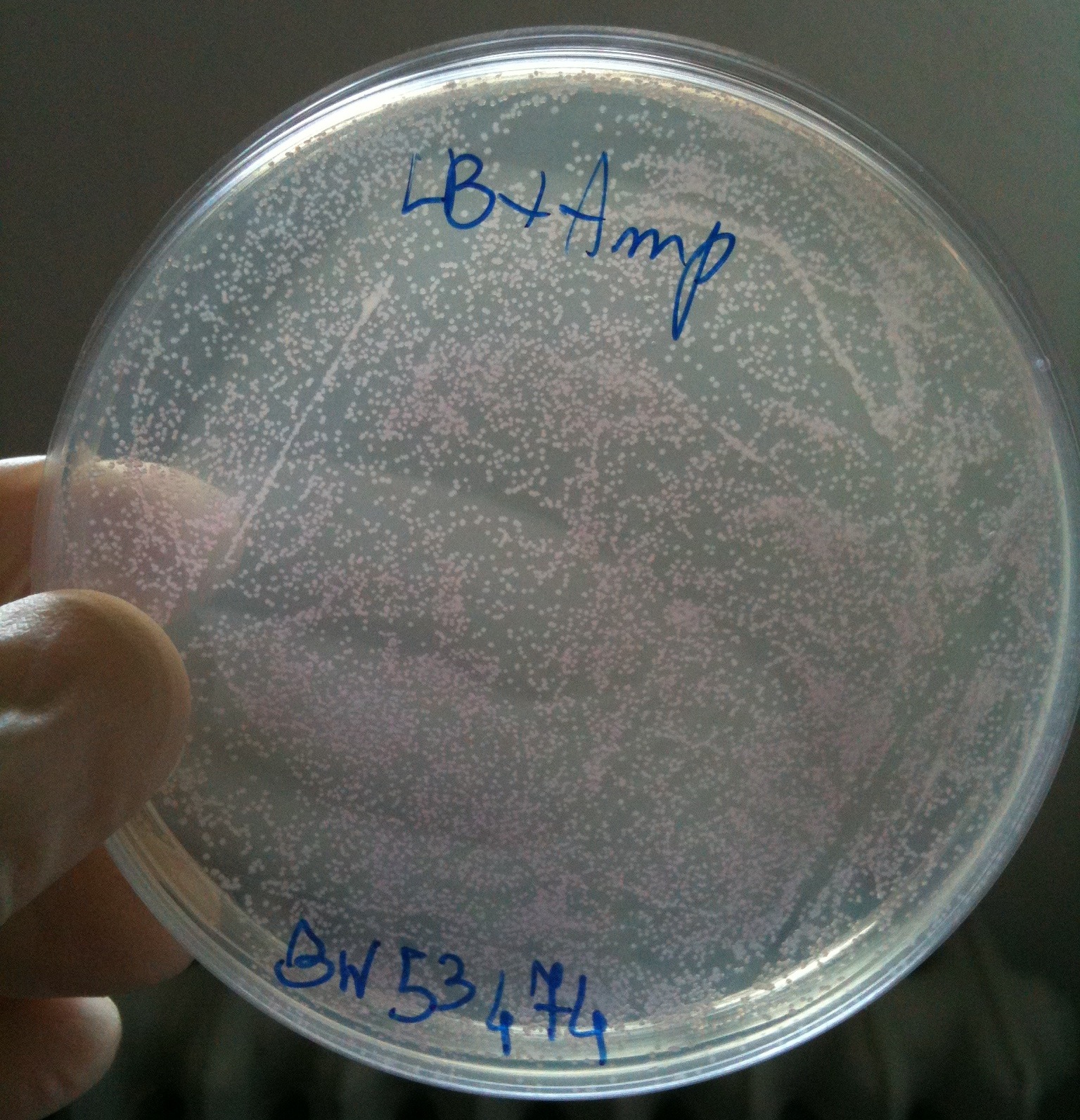
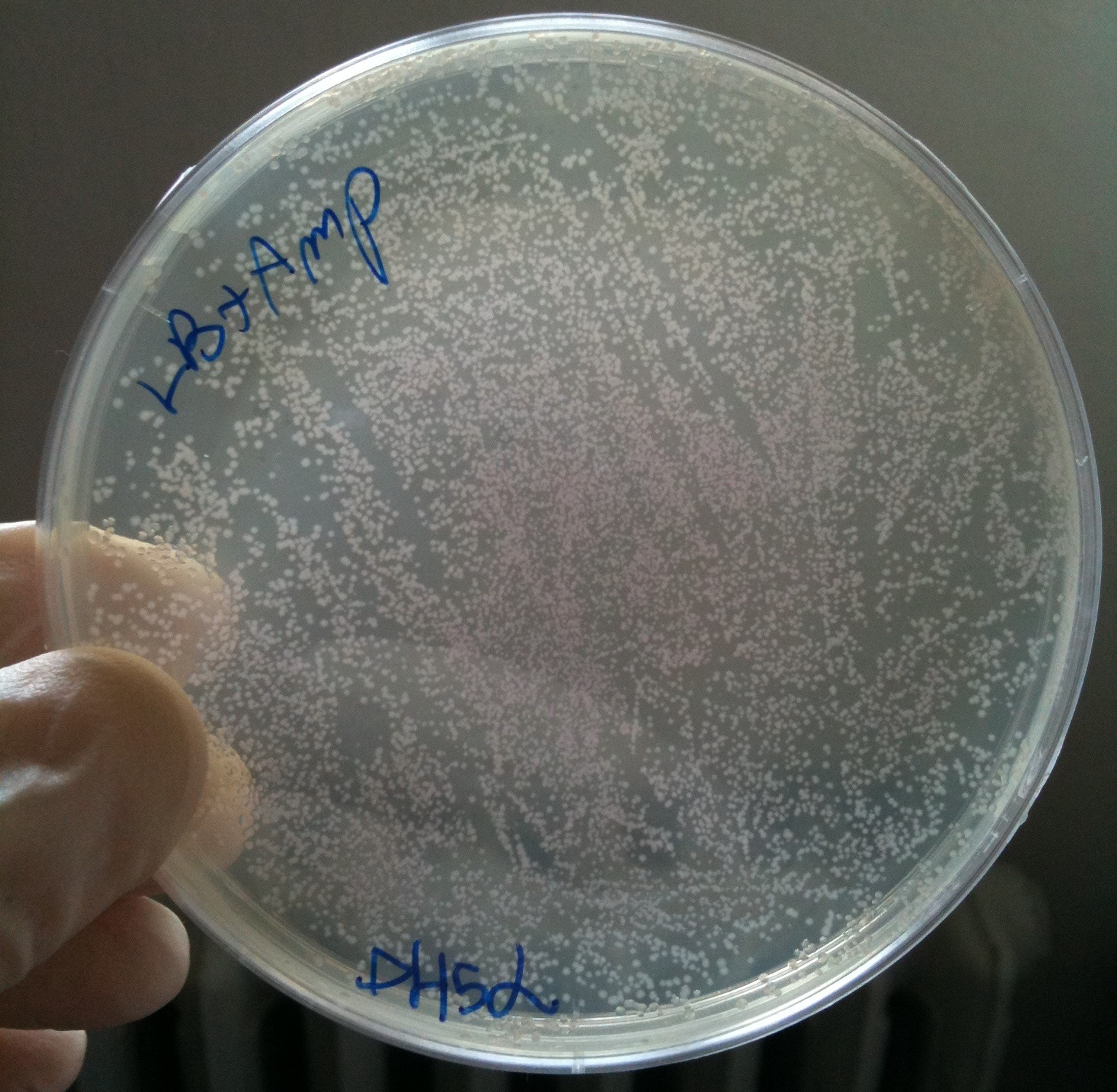
| - | + | We checked the presence of colonies in plates. | |
| + | All plates showed red colonies! | ||
| + | |||
| + | {|align="center" | ||
| + | | [[Image:MG_unipv10_efficiency.jpg|200px|thumb|center|MG1655 plate (with satellite colonies)]] || [[Image:BW25141_unipv10_efficiency.jpg|200px|thumb|center|BW25141 plate]] || [[Image:BW25142_unipv10_efficiency.jpg|200px|thumb|center|BW25142 plate]] | ||
| + | |- | ||
| + | |[[Image:BW53474_unipv10_efficiency.jpg|200px|thumb|center|BW53474 plate]] || [[Image:DH5-alpha_unipv10_efficiency.jpg|200px|thumb|center|DH5-alpha plate]] | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | We calculated (thanks Nicolò) efficiency as #colonies/ug DNA | ||
| + | {|border="1" align="center" | ||
| + | !Strain || #colonies || Efficiency | ||
| + | |- | ||
| + | | MG1655 || 379 || ~10^5 | ||
| + | |- | ||
| + | | BW25141 || 1431 || ~10^5 | ||
| + | |- | ||
| + | | BW25142 || 2374 || ~10^5 | ||
| + | |- | ||
| + | | BW53474 || 9984 || ~10^6<br/>(very difficult to count correctly) | ||
| + | |- | ||
| + | | DH5alpha || 7475<br/>(only 100ul of cells plated) || ~ 10^6<br/>(very difficult to count correctly,<br/>previous tests showed an efficiency of ~10^8) | ||
| + | |} | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| - | <table border="0" width="100%" | + | <!-- table previous next week --> |
| - | <tr | + | <br><br> |
| - | + | <table border="0" width="100%" class="menu"> | |
| - | < | + | <tr> |
| - | + | <td align="left">[[Team:UNIPV-Pavia/Calendar/July/settimana1| Previous week]]</td> | |
| - | + | <td align="right">[[Team:UNIPV-Pavia/Calendar/July/settimana3| Next week]]</td> | |
| - | + | </tr> | |
| - | + | ||
| - | [[Team:UNIPV-Pavia/Calendar/July/settimana1|week | + | |
| - | </td | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[Team:UNIPV-Pavia/Calendar/July/settimana3|week | + | |
| - | </td | + | |
| - | + | ||
| - | + | ||
| - | + | ||
</table> | </table> | ||
| + | <!-- fine table previous next week --> | ||
| + | </td> | ||
| + | <td width="15%" align="right" valign="top"> | ||
| + | {{UNIPV-Pavia/menu_mesi}} | ||
</td> | </td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
Latest revision as of 16:53, 24 October 2010
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"