Team:Wisconsin-Madison/delivery
From 2010.igem.org
Abstract
We have designed a universal platform for polypeptide release within the small intestine of the human gut that eliminates the need for any manual induction. Our automated, model system releases beta-galactosidase, a functional homologue of human lactase, once it reaches the duodenum to help a lactose intolerant patient metabolize lactose. The chassis for this system is the common probiotic in yoghurt, Lactobacillus acidophilus. Once the Lactobacillus acidophilus has reached the duodenum, they will lyse by either by a timed inducible/repressible system, a bile-inducible system, or an encryption system.
Background
Genetic Disorders
Lactose Intolerance
Physiology
Current Treatments
Probiotics
Modules
Enzyme Production
Our model system will tackle lactose intolerance. Producing Human Lactase in our bacterial cells would be difficult so we have chosen an enzyme that acts as functional homologue of human lactase called Beta-Galactacidase (B-Gal).
Left Lactase, Right Beta-Galactacide
We have taken advantage of the wealth of device that can be found in the iGEM Registry of Standard Parts. In 2008, the Cal Tech team had created a series of devices containing the gene for Beta-Galactacidase production. The difference in each device is the activity of each of the constitutive promoters. Thanks to their work, we can fine tune the amount of Beta-Galactacidase produces within our cells.
[IMAGE: RESULTS SHOWING CONSITUIVE EXPRESSION OF SERIES]
Capsule Basics
Our goals is to have each cell be surrounded by a protective 'capsule' to allow them to safely travel through the harsh acidic environment of the stomach to arrive in the small intestine for their main purpose. A past iGEM team used Transcription Factor RcsB to stimulate a the production of Colonic Acid from the capsule synthesis pathway of E.coli. Colonic Acid is a polymer of {=====] and has been shown to increase cell survivability in acidic conditions. We investigated the pathway for capsular polysaccharides synthesis and found more transcription factors that could give a similar or even better results!
[IMAGE: STOMACH INTESTINE]
RcsA and RcsB are transcription factors that are know to be positive regulators of capsular polysaccharides synthesis. We placed RcsA, RcsB, and a combination of the two under a IPTG inducible promoter to test both quantity of colonic acid produced and cell survivability. These two transcription factors form a heterodimer that is know to activate around 19 genes related to colonic acid synthesis. RcsB is also know to form a homodimmer and positively regulate cell division. RcsA and RcsB belong to the multicomponent RcsF/RcsC/RcsD/RcsA-RcsB phosphorelay system.
More information on these transcription factors and their usage in Biology can be found here: [Ecocyc-RcsA] & [Ecocyc-RcsB]
We wanted to test what IPTG induction levels were appropriate to protect our cells from an acidic environment of pH=2 before placing our devices under constitute control with appropriate ribosome binding sites.
[IMAGE OF 3 DEVICES] [CELL SURVIVABILITY] [COLONIC ACID #}\]
Based on our experiments quantifying the amount of colonic acid produces and cell survivability after acid shock, we have chosen [----------] to use in our final device which will be under constitutive promotion
Our final system will utililize a probiotic stain called Lactobacillus Acidophiles that is native to the human gut. The pathways in this bacterium are different than that of E.coli. Our next stage of research will involve finding a pathway, gene, or transcription factor that upregulates capsule synthesis and protocols to engineer this strain.
(IMAGE OF FINAL DEVICE]
One caution we had to consider in up-regulating this pathway is biofilm formation. Cholonic Acid synthesis is one of the first steps of the biofilm formation pathway. To ensure a biofilm is not produced we have taken advantage of an inhibitory transcription factor YgiV that blocks the activation of further steps in this pathway.
Lysis Expression
Why we need lysis.
pH Sensitive
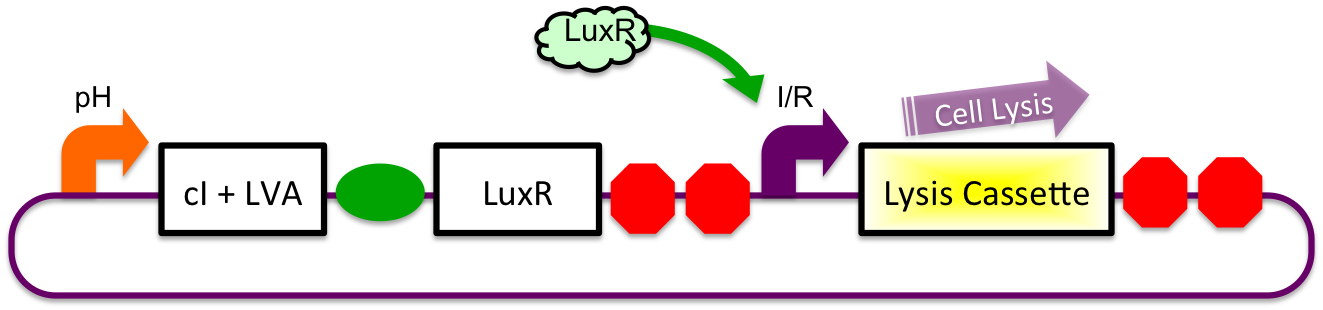
pH sensitive expression of LuxR and cI-LVA proteins will be accomplished via the glutamate decarboxylase A promoter (gadAp). Native to the E. coli acid resistance "island", regulation of expression from this promoter is normally accomplished by binding or unbinding of the Fis, GadX, and Crp proteins. Although the mechanism and exact conditions which bring about expression from the promoter are not well understood, studies have pointed to a combination of stationary phase and low environmental pH causing induction (Castanie-Cornet MP et al, 2001; 2010).
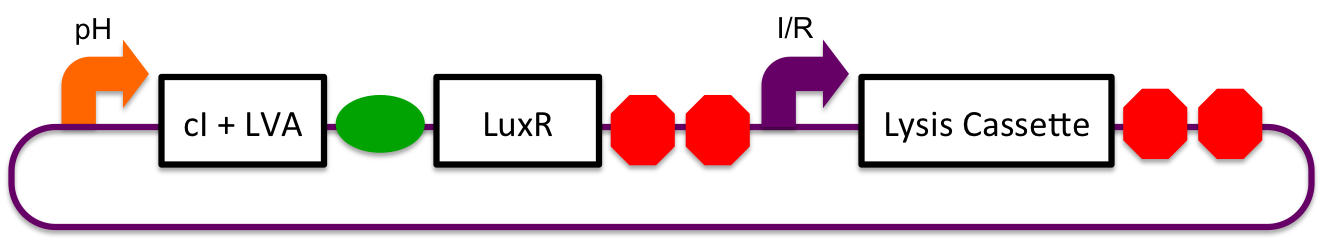
Inducible-Repressible
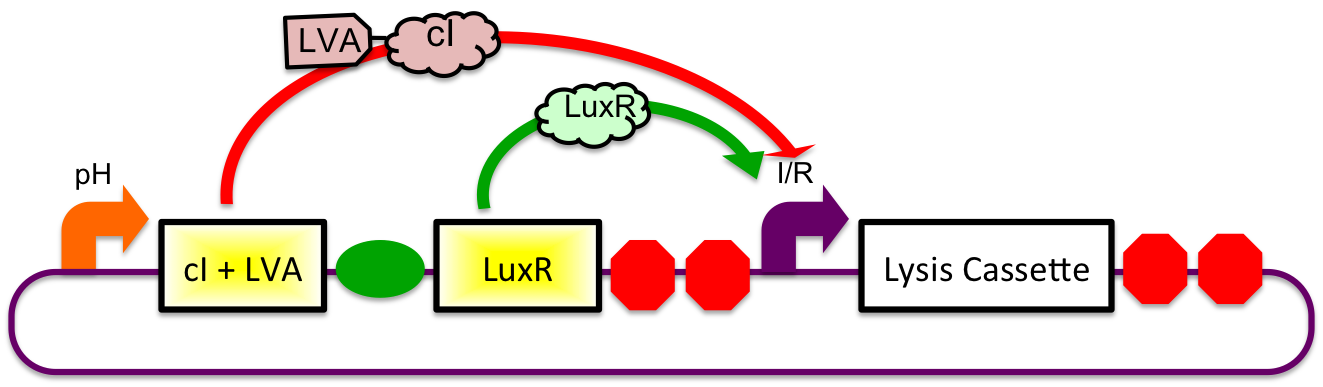
A previously characterized inducible-repressible promoter (BBa_R0065) will be used to accomplish our goal of delivering E. coli lactase to the human intestine. This system will be used in combination with the previously described gadA promoter, LuxR to yield expression after the active cell has seen the acidic conditions of the stomach. In order to better describe the order of events, refer to the following diagrams and descriptions:
1) Before Ingestion The gadA promoter sees marginally acidic or neutral conditions and therefore yields expression of insignificant levels of both LuxR and cI-LVA. Since expression of both LuxR and cI-LVA are at low levels, the level of induction from inducible-repressible
2) In Stomach
3) Immediately After Stomach
4) In Small Intestine
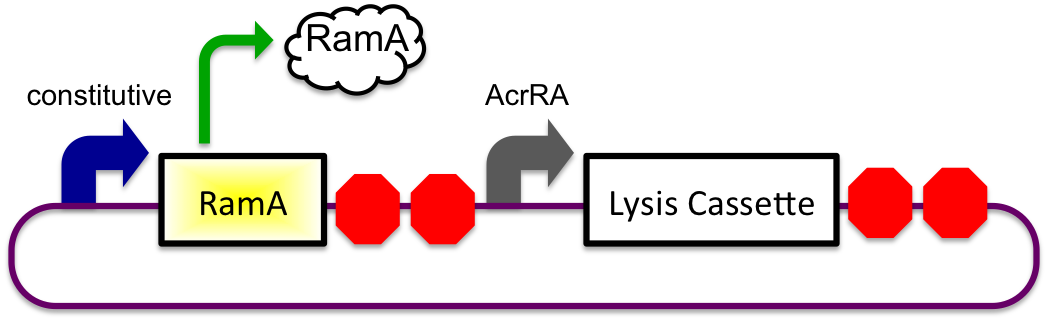
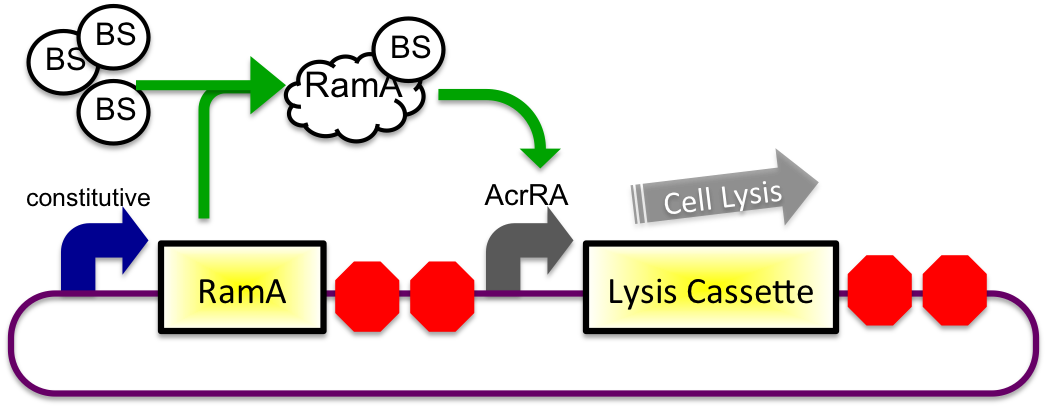
Bile Sensitive
Explain system here
1) Before Small Intestine
2) In Small Intestine
Enzyme Action
Complete System
Significance
Rare Genetic Disorders
Biopharmaceuticals
Protein purification
Enzyme Activity
Industry
Fermentation
Business Plan
Cost Effectiveness
Animation
 "
"