Team:Valencia/Safety
From 2010.igem.org
Alejovigno (Talk | contribs) |
Alejovigno (Talk | contribs) |
||
| Line 1: | Line 1: | ||
| + | <!-- | ||
| + | |||
{{:Team:Valencia/head}} | {{:Team:Valencia/head}} | ||
| Line 5: | Line 7: | ||
Please use this page to answer the safety questions posed on the [[Safety | safety page]]. | Please use this page to answer the safety questions posed on the [[Safety | safety page]]. | ||
| - | </div> | + | </div> --> |
| + | {{:Team:Valencia/cabecera3}} | ||
| + | ====Sup35p==== | ||
| + | [PSI+] is a non-mendelian trait of ''Saccharomyces cerevisae'' that supress nonsense codons. This phenotype is due to a self-replication conformations of the protein Sup35p, a translation-termination factor. In [psi-] cells, the translation-termination factor Sup35 is soluble and functions with Sup45 to recognize stop codons and terminate translation. | ||
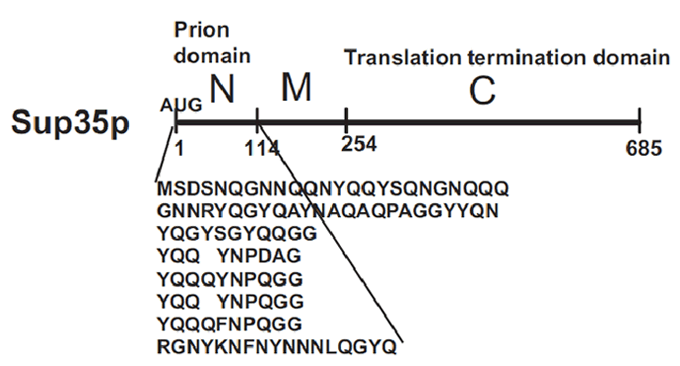
| + | [[Image:Valencia_prion_sup35.gif|thumb|400px|The prion domain of Sup35 is Q/N Rich and has the ability to propogate the corresponding prion in the absence of the rest of the molecule. Source: Wickner ''et al.'' 2008.]] | ||
| + | In [PSI+] cells, most Sup35 is insoluble and nonfunctional, causing a reduction on translation fidelity. This trait is heritable because Sup35 protein in the [PSI+] conformation influences new Sup35 protein to adopt the same conformation and passes from mother cell to daughter to perpetuate the cycle of conversion. [PSI+] is, however, metaestable: [PSI+] cells occasionally give rise to [psi-] cells and viceversa, as the [PSI+] conformation is lost and gained. | ||
Revision as of 08:29, 22 October 2010
Sup35p
[PSI+] is a non-mendelian trait of Saccharomyces cerevisae that supress nonsense codons. This phenotype is due to a self-replication conformations of the protein Sup35p, a translation-termination factor. In [psi-] cells, the translation-termination factor Sup35 is soluble and functions with Sup45 to recognize stop codons and terminate translation.
In [PSI+] cells, most Sup35 is insoluble and nonfunctional, causing a reduction on translation fidelity. This trait is heritable because Sup35 protein in the [PSI+] conformation influences new Sup35 protein to adopt the same conformation and passes from mother cell to daughter to perpetuate the cycle of conversion. [PSI+] is, however, metaestable: [PSI+] cells occasionally give rise to [psi-] cells and viceversa, as the [PSI+] conformation is lost and gained.
 "
"