|
|
| (18 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | {{Team:USTC_Software/Header}} | | {{Team:USTC_Software/Header}} |
| - | | + | {| cellpadding="15" cellspacing="0" |
| - | Using C-N Model we can model systems with great complexity
| + | |
| - | ==The simplest example with only E.coli cell==
| + | |
| - | | + | |
| - | ===introduction===
| + | |
| - | As the simplest example, a model of a flask with only E.coli cells inside will be shown. Only one reaction will be considered: the replication of E.coli. However, it is not trivial. '''MoDeL''' enables users to add such an auto-catalytic replication reaction easily and conveniently. The minimal database used for this model could be download here.
| + | |
| - | | + | |
| - | ===database construction===
| + | |
| - | We only provide key points regarded with construction of this minimal database. To add the auto-catalytic reaction in the Reaction container, a species with only part E.coli is required. It has the simplest '''Chain-Node''' model format: only one chain with one part and no trees. The auto-catalytic reaction has one modifier and one product and they are both referred to E.coli defined in Species container. Since they are compartment-type species (species representing a compartment), attribute '''itself''' of '''compartmentLabel''' node in modifiers and products definitions should be set the same with label of the compartment they represent in the compartments definition. It ensures that the product and the modifier are the same, avoiding wrong mismatch of the product which is different with the modifier. Since number of E.coli cells will reach a stable level in a long time course, we use <math>k_{g}(1-C_{E.coli}/C_{max})C_{E.coli}V_{Flask}</math> as the reaction rate, where <math>k_{g}</math> is the growth rate of E.coli, <math>V_{Flask}</math> represents the size of E.coli, and <math>C_{E.coli}</math> and <math>
| + | |
| - | C_{max}</math> are concentration of E.coli and its max concentration in the flask, respectively. The negative sign in the rate equation indicates self-repression of E.coli cells.
| + | |
| - | | + | |
| - | ===modelling and simulation===
| + | |
| - | [[Image:Ustcs Graph1.png|400px|thumb|Replication of E.coli cell]]
| + | |
| - | Model written in '''SBML''' format is shown below. The dynamic curve of E.coli replication is plotted by time course simulation. Exactly speaking, dynamic behaviors of biological system are meaningless after 4000s because of termination of reproduction of E.coli. However, the curve is in qualitative agreement with that from experiments in the first and second stage of reproduction of E.coli.
| + | |
| - | | + | |
| - | ==Basic example with pLac-LacI repression system==
| + | |
| - | | + | |
| - | ===introduction===
| + | |
| - | '''MoDeL''' provides poweful supports for description of interactions between two species. No interactions exist in the previous example. In this example, we will construct a plasmid backbone with promoter, rbs, coding sequence and terminator inserted in and transform it into E.coli. LacI repressor are produced after two steps of transcription and translation. It binds with itself to form a dimer and then a tetramer, which could bind with promoter LacI on the plasmid to repress the process of transcription. Besides, replication of E.coli cells, degradation of proteins and mRNAs, as well as dilution due to replication of cells, are also considered.
| + | |
| - | | + | |
| - | ===database construction===
| + | |
| - | Actually, it is a complex molecular process and takes many steps to complete a transcription reaction. It includes binding and initiation, elongation, and termination. So does translation reactions. The first version of '''MoDeL''' '''<font color='red'>DOES NOT</font>''' support transcription and translation reaction templates and they will be handled by our core program of '''iGame''' and thus no need to write in the database.
| + | |
| - | | + | |
| - | All binding reactions in the database demo are modeled using law of mass action. They are dimerization of two LacI repressors and that of two LacI dimers. LacI tetramers will bind with promoter LacI in the plasmid and thus repressing the expression of plasmid DNAs. However, '''MoDeL''' enables any mathematical model form for species-species interactions and is not constrained within only law of mass action. Users could change the reactants, modifiers, products, as well as kinetic laws arbituarily and validate their models via output of dynamic curves.
| + | |
| - | | + | |
| - | Species reaction templates are worth mentioning to show features of our new language '''MoDeL'''. It is never a easy problem to calculate degradation rates of proteins since different parts in a fused protein may have different degradation rates and the overall rate is still unknown. What's more, a protein in '''MoDeL''' may have different protein chains and it is more complex to determine the overall rate. In the demo database, we assume all proteins with no parts in bound state have a uniform degradation rate. Proteins with more than one chains are considered stable and non-degradable. This idea could be applied easily by writing reaction templates in '''MoDeL''' format. The reactant is a general species template with only one substituent-type part ('''ANYUB''') of type '''ForwardProtein''' (no reverse reactions happened here). It is similar for degradation of mRNA molecules. Only mRNAs with single chain degrades at the same rate.
| + | |
| - | | + | |
| - | For reactions of dilution due to reproduction of cells, quantities of species decrease in a similar way to degradation. The rate of dilution is also similar to the reproduction rate of E.coli:
| + | |
| - | | + | |
| - | <math>k_{g}(1-C_{E.coli}/C_{max})C_{species}V_{Flask}</math>
| + | |
| - | | + | |
| - | where we only replace the second <math>C_{E.coli}</math> with concentration of species in dilution. To apply this idea, we only need to construct a species with only one substituent-type part (''ANY'') of no specific type and it will be matched to all species. Among them, only species bound in compartment E.coli will be diluted.
| + | |
| - | | + | |
| - | Replication of plasmid DNAs is not as easy as reproduction of living cells in two aspects: first, plasmid backbone with the same replication origin could be found in many species in the system because the original transformed plasmid backbone may bind with other compounds or proteins to form complex; second, the replication of original transformed plasmid DNA molecule tends to be repressed by all species including the same replication origin. However, E.coli cells do not have any effects on the reproduction of other E.coli cells. '''MoDeL''' language perfectly support this idea by separating the template reaction into replications and repressions. The first part describes the exponential amplification of plasmids with reaction rate <math>k_{g}C_{plasmid}V_{E.coli}</math>, where <math>k_{g}</math> is the replication rate of a certain plasmid, <math>C_{plasmid}</math> is the concentration of this plasmid, and <math>V_{E.coli}</math> is the volume of E.coli cell. The product may be different from the reactant. It is possible because the reactant may be a complex, such as complex of LacI tetramer and plasmid with promoter LacI (we call it pLacI:LacI4 below). Only the products of the replication reaction will be repressed with a certain rate.
| + | |
| - | In this example, pLacI:LacI4 would not be repressed since it is not among the products of its replication. Via '''MoDeL''' language, we design a template reaction with no reactants, two modifiers and one product. The two modifiers are template species containing a certain plasmid backbone (say pSB1A3), and binding state is allowed for both. The product is also a template species containing pSB1A3, however, it has only one chain and no binding is permitted. Hence, modifiers have '''Chain-Node''' structure ''ANY''--pSB1A3--''ANY'', while product has its structure ''ANYUB''--pSB1A3--''ANYUB''. The ''listOfTransferTable'' makes parts transfer from part ''ANY'' of either modifier to its corresponding part ''ANYUB'' of the product. The reaction rate is <math>-k_{g}C_{mod1}C_{mod2}/C_{max}</math>, where <math>k_{g}</math> is the same as that defined in the replication reaction, <math>C_{max}</math> is the max concentration of plasmids containing pSB1A3 as backbone, and <math>C_{mod1}</math>, <math>C_{mod2}</math> are concentrations of modifier1 and modifier2, respectively. The negative sign in the rate law indicates a repressed effect to the replication of the product.
| + | |
| - | | + | |
| - | ===modelling and simulation===
| + | |
| - | {| cellpadding="10" cellspacing="0" | + | |
| | |- | | |- |
| - | |Only one copy of plasmid is transformed into E.coli cell at initial time. To give a reasonable dynamic curve of species in the biological system, parameters are obtained from experiments if they are available. Besides E.coli, there are 6 species: DNA with pLacI and LacI (DNA0), LacI mRNA (with RBS), LacI protein, LacI dimer, LacI tetramer, and complex of LacI tetramer and DNA0 (DNA1). Concentrations of LacI mRNA, protein, dimer and tetramer versus time are plotted in Figure 4. Since dissociation constant (Kd) of LacI-LacI monomer interaction (77nM) is mush larger than that of LacI dimer-dimer interaction (0.1pM), steady-state concentrations of LacI mRNA and protein are in different scales with that of LacI dimer and tetramer. Due to very large Kd of LacI dimer-dimer interaction, LacI protein would finally exist in form of tetramer in the system (concentration of LacI dimer is several orders of magnitude lower than the scale of the left Y axis and thus be ignored). | + | !|<font size="5">[[Team:USTC_Software/Transfer%20function%20of%20Part:BBa_F2620|pLux-LuxR repression system]]</font> |
| - | |[[Image:Ustcs plac lacI 1.png|400px|thumb|Figure 4: Dynamic curves of LacI mRNA, protein, dimer and tetramer]] | + | !|<font size="5">[[Team:USTC_Software/Toggle-Switch|Toggle-Switch]]</font> |
| | |- | | |- |
| - | |Figure 5 shows dynamic behaviors of plasmids in our model. Since only one copy of plasmid DNA0 was transformed, its quantity increases at the very beginning and decrease soon because all DNA0 will bind with LacI tetramer and no free plasmid exists. The process will end in one hour and quantities of both DNA0 and DNA1 enter into a stable level. It is worth mentioning that the stable concentration of DNA1 is about 380 nM, a little lower than its maximum concentration 475 nM. It is owing to reproduction of E.coli cells. After quantities of E.coli cell go into its stable stage, its concentration will continue to increase until the maximum setup value. | + | |valign="top"|[[Image:USTCs luxr gfp assembly.PNG|400px|thumb|center]] |
| - | |rowspan="2"|[[Image:Ustcs plac lacI 2.png|400px|thumb|Figure 5: Dynamic curves of DNA0 and DNA1]] | + | |valign="top"|[[Image:USTCs Toggle assembly.PNG|400px|thumb|center]] |
| | |- | | |- |
| - | |This basic example proves great success for '''iGAME''' to automatically model genetic regulatory systems. It shows real biological process in a qualitative manner and give suggestions for users to adjust their experiment plans. | + | !|<font size="5">[[Team:USTC_Software/Repressilator|Repressilator]]</font> |
| | + | |- |
| | + | |valign="top"|[[Image:USTCs Oscillator assembling.PNG|400px|thumb|center]] |
| | |} | | |} |
| - |
| |
| - | ==Transfer function of Part:BBa_F2620==
| |
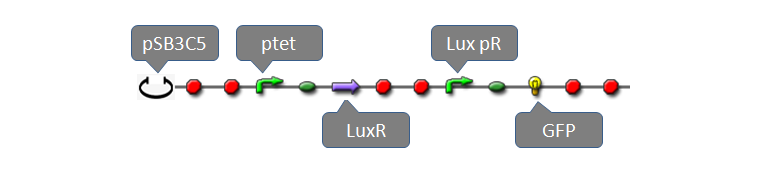
| - | Part:BBa_F2620 had been fully characterized with data in partsregistry [http://partsregistry.org/Part:BBa_F2620|page]. It is a composite biobrick with three individual biobricks: pTetR (R0040), LuxR(C0062) and lux pR. Without AHL, transcriptional level of lux pR is extremal low because of lack of activator, LuxR-AHL dimer. If we add AHL into the system within a very short time, expression of mature GFP is expected to increase significantly. To measure behaviors of the system consisting of BBa_F2620 using our software tool, we construct a system using plasmid pSB3C5 and Part:BBa_F2620 as well as a reporter gene:
| |
| - |
| |
| - | [[Image:USTCs_luxr_gfp_assembly.PNG|900px|center]]
| |
| - |
| |
| - | where we use two consecutive terminators to indicate a complete termination. Time course simulation was performed to generate transfer function of stable GFP concentrations versus AHL concentration. It is of high consistence with experiment done by [http://partsregistry.org/Part:BBa_F2620:Transfer_Function|Haseloff Lab, MIT]:
| |
| - |
| |
| - | [[Image:USTCs Transfer function.png|900px|center]]
| |
| - |
| |
| - | where GFP concentration is directly proportional to its synthetic rate. In our simulation, we add AHL of concentration ranging from 1E-10 to 1E-5 M (increasing by order of magnitude) to the reactor within one minute. Details of modeling are described [[here]].
| |
| - |
| |
| - | We also plot time and dose response measurements of GFP stable concentration following addition of AHL. We choose the same AHL concentrations as done in testing the transfer function and plot their dynamic curves of GFP:
| |
| - |
| |
| - | [[Image:USTC_s_dose_response.PNG|900px|center]]
| |
| - |
| |
| - | ==Toggle Switch==
| |
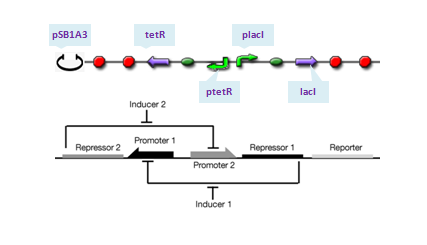
| - | The toggle switch is composed of two repressors and two promoters, each of which is inhibited by the repressor transcribed by the other promoter. The parts assembling is as Figure 1 in [1]. We choose pLacI and pTetR as the two promoters, and the repressors are TetR and LacI, respectively.Theoretically, if ratio of LacI to TetR is greater than 1, strong binding of LacI to pLacI repress transcription initiation from pLacI and therefore quantities of TetR will decrease, which in turn relieves its repression to pTet leading to increase of quantities of LacI itself. In this situation, concentration of LacI is far greater than that of tetR, whose expression is repressed at an extream low level and the system enters into its 'LacI' state. Similar analysis will do for TetR and the system will enter into 'TetR' state if its quantity dominates. To show transition between the two states, IPTG is added in 1s after the system enters into 'LacI' state completely.
| |
| - |
| |
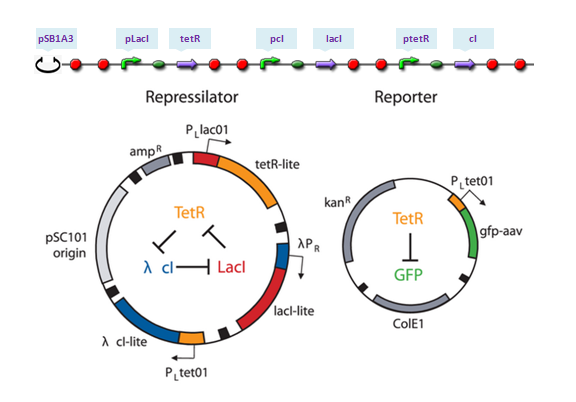
| - | ==Repressilator==
| |
| - | The repressilator is a synthetic genetic regulatory network designed to exhibit a stable oscillation shown via expression of GFP (green fluorescent protein). The work is reported by Michael B. Elowitz and Stanislas Leibler in their [http://www.nature.com/nature/journal/v403/n6767/full/403335a0.html work] at 2000. They constructed a system of three genes connected in a cyclical negative feedback loop so that gene A represses gene B, which represses gene C, which represses gene A. The implementation of this idea used a low copy plasmid encoding the repressilator, and the higher copy reporter, which were used to transform a culture of Escherichia coli[http://en.wikipedia.org/wiki/Repressilator].
| |
| - | [[Image:USTCs_Repressilator_plasmid.png|400px|center]]
| |
| - |
| |
| - | LacI binds to form dimer and LacI dimer will continue to bind to form LacI tetramer, which acts as the repressor of pLacI gene. Meanwhile, TetR and cI only dimerize. Repressors of pTetR and pCI genes are TetR and cI dimers, respectively.
| |
| - |
| |
| - | To design a database specially used for this classical system, we model the
| |
| - |
| |
| - | ==Synchronized genetic oscillators==
| |
| - | ==E. coli Automatic Directed Evolution Machine==
| |
| - | The strength of promoters used in the model, characterized by attribute ''forwardPromoterEfficiency'' could be obtained [[Team:USTC_Software/downloads/ADEM/parameter#Characterization_of_activities_of_promoters|here]].
| |
 "
"