Team:UNIPV-Pavia/Project/solution
From 2010.igem.org
m |
(→Integrative standard vector for yeast) |
||
| Line 228: | Line 228: | ||
=Integrative standard vector for yeast= | =Integrative standard vector for yeast= | ||
| + | |||
| + | Here, a detailed description of the integrative vector for the yeast ''S. cerevisiae'' is reported. | ||
| + | |||
| + | The structure of the designed vector, here named BBa_K300001, is shown in Fig.1. Most of its features have been inspired by the pUG6 plasmid (GenBank: AF298793.1), constructed by [Guldener U et al., 1996]. | ||
| + | |||
| + | {|align=center | ||
| + | |valign=top|[[Image:k300001base.jpg|thumb|500px|center|Figure 1: BioBrick integrative base vector BBa_K300001.]] | ||
| + | |[[Image:glossary300001.jpg|thumb|300px|center|Figure 2: Parts notation.]] | ||
| + | |} | ||
| + | |||
| + | This is an integrative vector which can be used to insert the desired RFC10-compatible BioBrick parts/devices/systems into the genome of ''S. cerevisiae''. This vector can also be specialized to target the desired integration site in the host genome. | ||
| + | |||
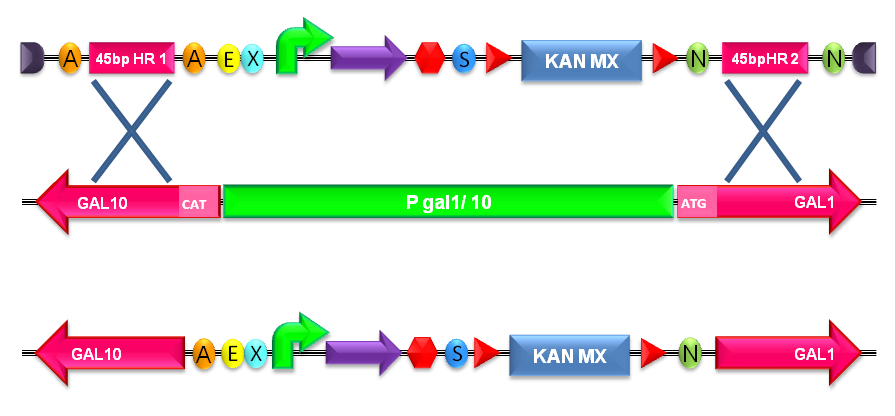
| + | The default version of this backbone targets the Gal system of the S288C strain (<partinfo>BBa_K300979</partinfo>) through the two homologous regions <partinfo>BBa_K300986</partinfo> and <partinfo>BBa_K300987</partinfo>. The Gal system is not essential for yeast survival if the strain is grown on carbon sources other than galactose. | ||
| + | |||
| + | This vector enables multiple integrations in different positions of the same genome. The usage of the KanMX dominant selection marker can avoid the usage of auxotrophic markers. In the industrial framework auxotrophies are usually deleterious for the process productivity because they affect the growth rate of cells. For this reason, this vector can be a concrete solution for the design of industrial yeast strains with novel user-defined functions. | ||
| + | |||
| + | |||
| + | ===Glossary=== | ||
| + | '''HR''' (Homologous Region) is a sequence that can recombine with the host genome. | ||
| + | |||
| + | |||
| + | ===How to propagate it before performing genome integration=== | ||
| + | This vector can be easily propagated in ''E. coli'' thanks to the high-copy replication origin and the Ampicillin resistance selection marker, both derived from the <partinfo>pSB1A2</partinfo> vector backbone. | ||
| + | |||
| + | |||
| + | ===How to integrate a BioBrick into the yeast genome=== | ||
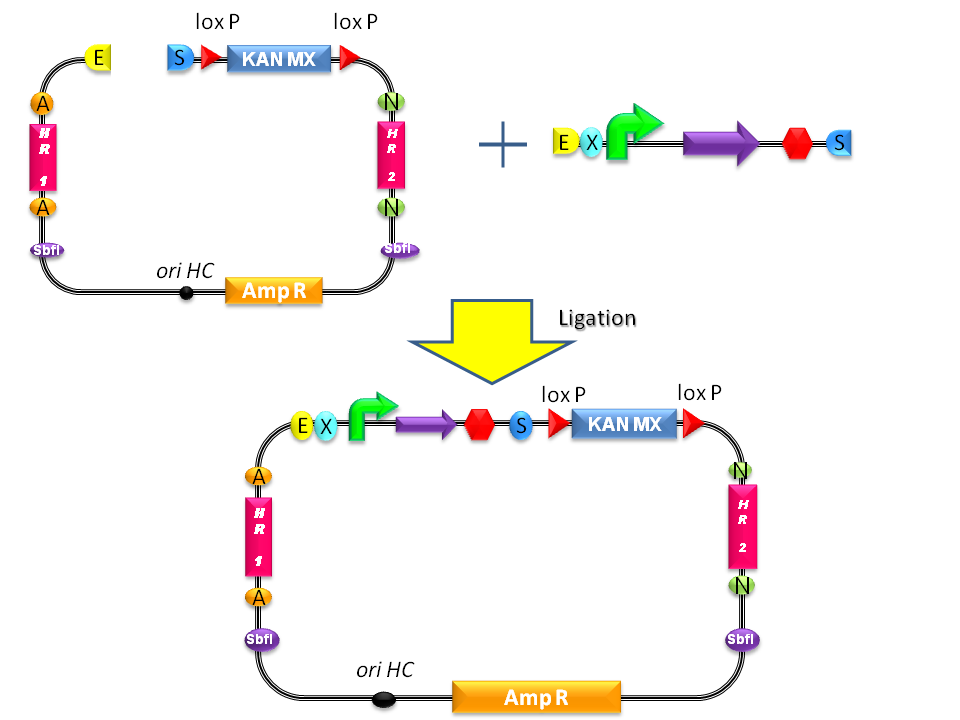
| + | #Digest <partinfo>BBa_K300001</partinfo> and the desired BioBrick part with EcoRI-SpeI and ligate them (Fig.1-a). | ||
| + | #Propagate the resulting plasmid in ''E. coli'' and extract plasmid DNA from bacteria. | ||
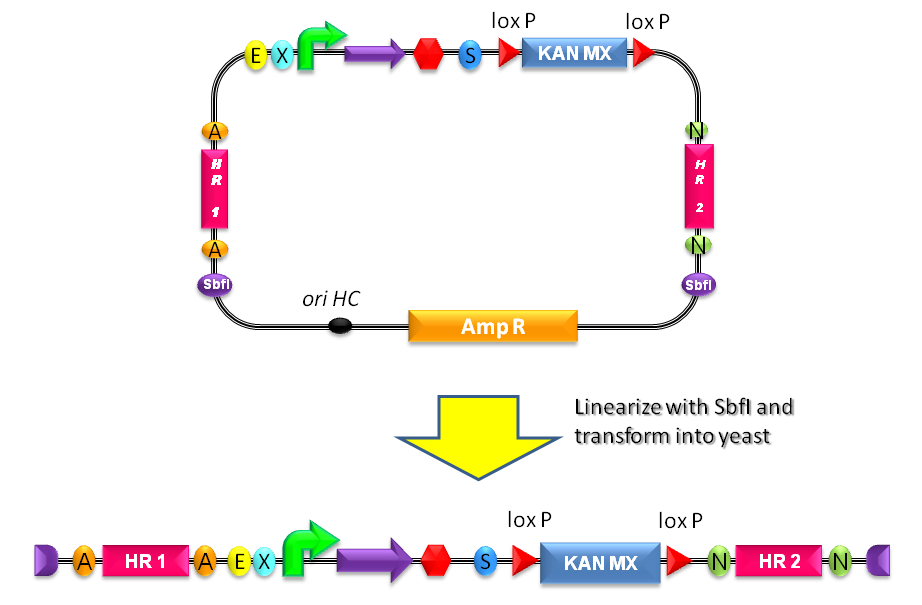
| + | #Digest the resulting plasmid with SbfI to linearize the DNA of interest (Fig.1-b). This is known to increase the integration efficiency from 10- to 50-fold when compared to a non-linearized DNA [http://partsregistry.org/Part:BBa_K300001:Design#References Reference 11]. | ||
| + | #Transform the linearized plasmid into ''S. cerevisiae'' and select integrants on G418 antibiotic plates (Fig.1-c and d). | ||
| + | |||
| + | {|align=center | ||
| + | |[[Image:k300001.jpg|thumb|500px|center|Figure 3: Assembly of the desired BioBrick part into the integrative vector.]] | ||
| + | |} | ||
| + | |||
| + | {|align=center | ||
| + | |[[Image:linear300001.jpg|thumb|600px|center|Figure 4: The plasmid can be linearized upon SbfI digestion to separate the yeast DNA fragment (containing the part of interest and the LoxP-KanMX-LoxP cassette) from the ''E. coli'' DNA fragment (containing the replication origin and the Amp resistance).]] | ||
| + | |} | ||
| + | |||
| + | {|align=center | ||
| + | |[[Image:recomb300001.jpg|thumb|600px|center|Figure 5: Integration of the BioBrick of interest into the ''S. cerevisiae'' genome by homologous recombination. In this figure, the integration in the Gal system genomic region is shown.]] | ||
| + | |} | ||
| + | |||
| + | Users can change the integration site by engineering the vector: <partinfo>BBa_K300986</partinfo> and <partinfo>BBa_K300987</partinfo> are flanked by two AvrII and two NheI respectively and for this reason the two Homologous Regions can be excided. New homologous sequences compatible with RFC10 can be digested with XbaI-SpeI and assembled because AvrII, NheI, XbaI and SpeI have compatible sticky ends. Note that this assembly is not directional and the correct orientation can be validated through sequencing with standard VF2 and VR primers. | ||
| + | |||
| + | |||
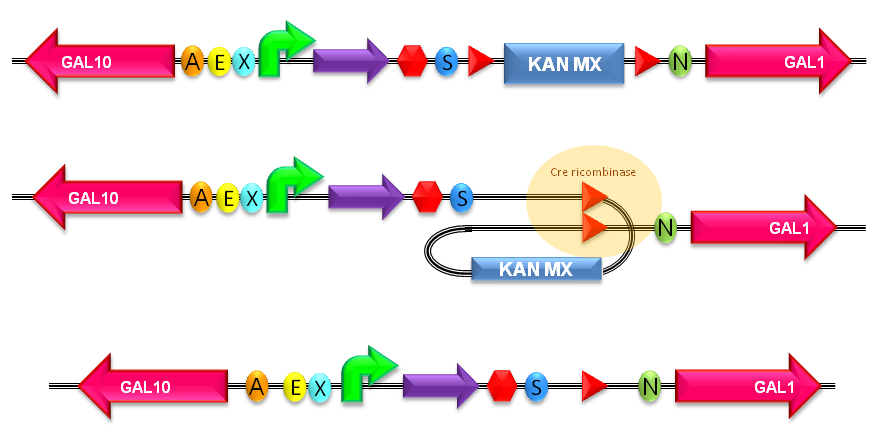
| + | ===How to perform the KanMX marker excision=== | ||
| + | The KanMX dominant selection marker is flanked by two loxP recombination sites and for this reason it can be excided upon Cre recombinase activity. The Cre recombinase has to be expressed by a helper plasmid. | ||
| + | |||
| + | |||
| + | {|align=center | ||
| + | |[[Image:loxp300001.jpg|thumb|600px|center|Figure 6: KanMX excision with loxP sites recombination. For more details about this process, see http://partsregistry.org/Recombination]] | ||
| + | |} | ||
| + | |||
| + | |||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| Line 233: | Line 288: | ||
<img src="https://static.igem.org/mediawiki/2010/7/74/UNIPV_Pavia_Icona_Bioplastica.GIF" width="75px" height="75px"/></a> | <img src="https://static.igem.org/mediawiki/2010/7/74/UNIPV_Pavia_Icona_Bioplastica.GIF" width="75px" height="75px"/></a> | ||
</html> | </html> | ||
| + | |||
=Self-cleaving affinity tags to easily purify proteins= | =Self-cleaving affinity tags to easily purify proteins= | ||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
Revision as of 15:28, 23 October 2010
|
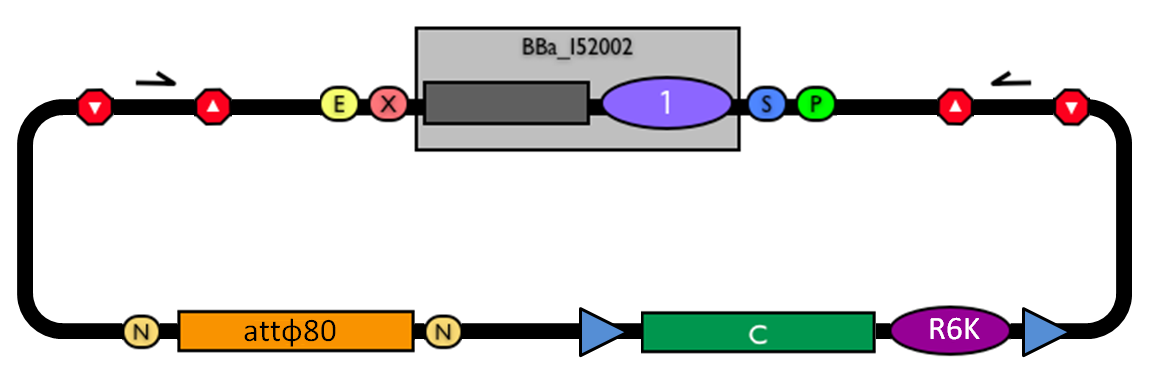
This vector can be considered as a base vector, which can be specialized to target the desired integration site in the host genome. The default version of this backbone has the bacteriophage Phi80 attP (<partinfo>BBa_K300991</partinfo>) as integration site. This vector enables multiple integrations in different positions of the same genome.
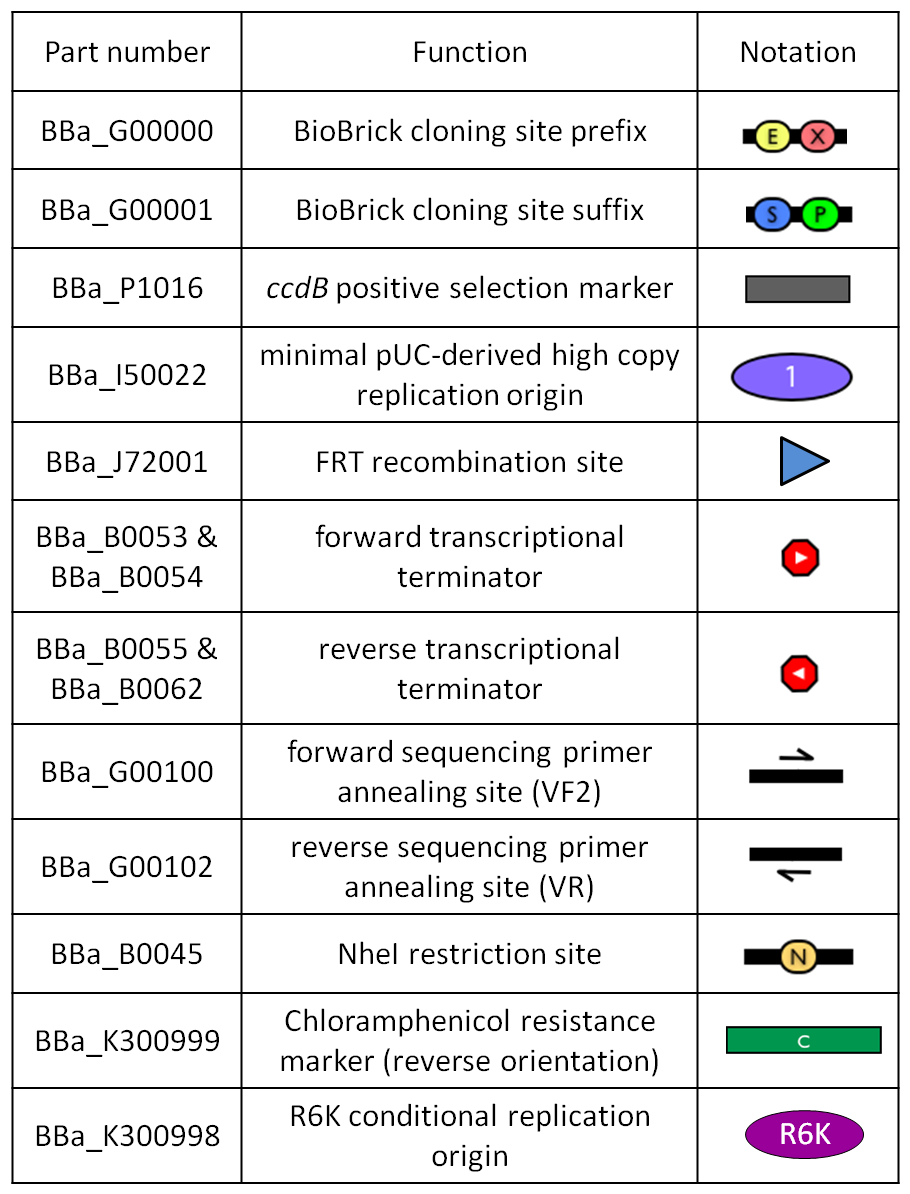
GlossaryThe passenger is the desired DNA part to be integrated into the genome. The guide is the DNA sequence that is used to target the passenger into a specific locus in the genome.
The main design features for vector engineering and for the genome integration of the vector are reported below. Vector engineering features:
Genome integration features:
How to use it<partinfo>BBa_K300000</partinfo> can be:
How to propagate it before performing genome integrationThe default version of this vector contains the <partinfo>BBa_I52002</partinfo> insert, so it *must* be propagated in a ccdB-tolerant strain such as DB3.1 (<partinfo>BBa_V1005</partinfo>). After the insertion of the desired BioBrick part in the cloning site, this vector does not contain a standard replication origin anymore, so it *must* be propagated in a pir+ or pir-116 strain such as BW25141 (<partinfo>BBa_K300984</partinfo>) or BW23474 (<partinfo>BBa_K300985</partinfo>) that can replicate the R6K conditional origin (<partinfo>BBa_J61001</partinfo>).
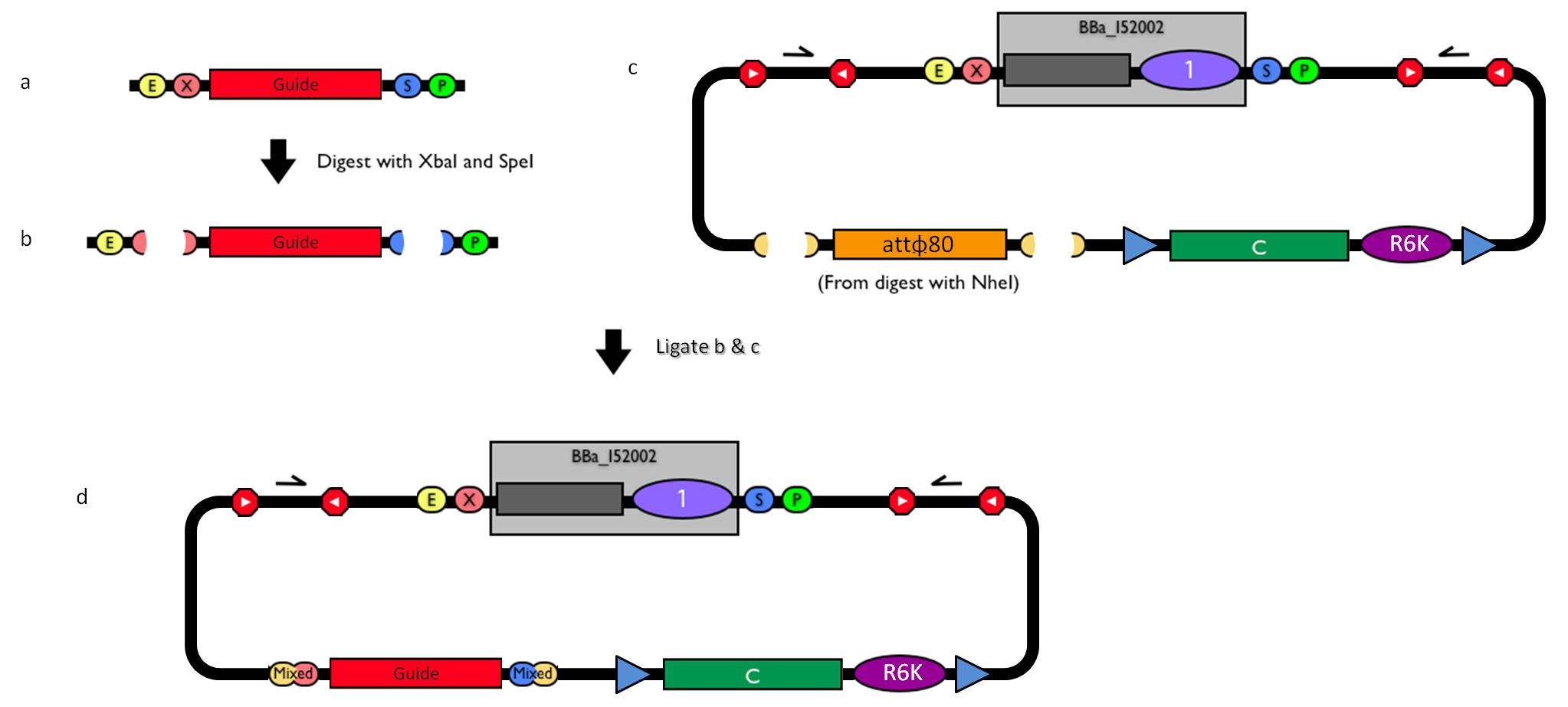
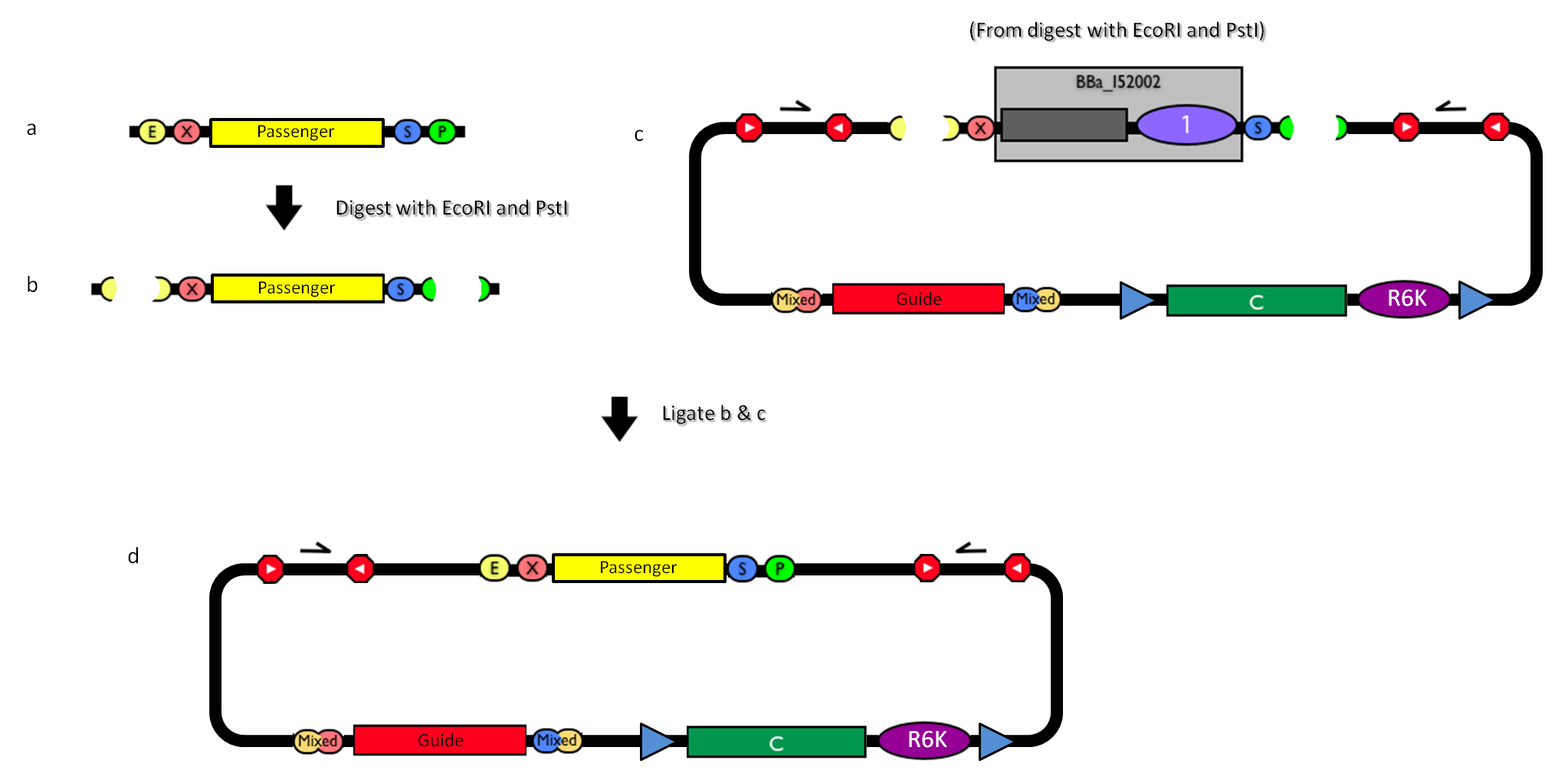
How to engineer itThe DNA guide can be changed as follows:
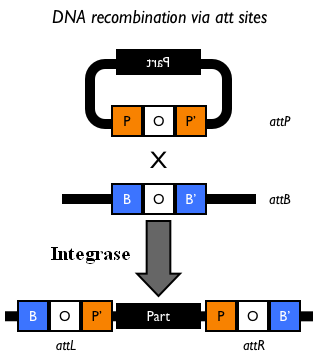
How to perform genome integrationThe integration into the E. coli chromosome can exploit the bacteriophage attP-mediated integration or the homologous recombination. Detailed protocols about attP-mediated integration can be found here:
Detailed protocols about homologous recombination can be found here:
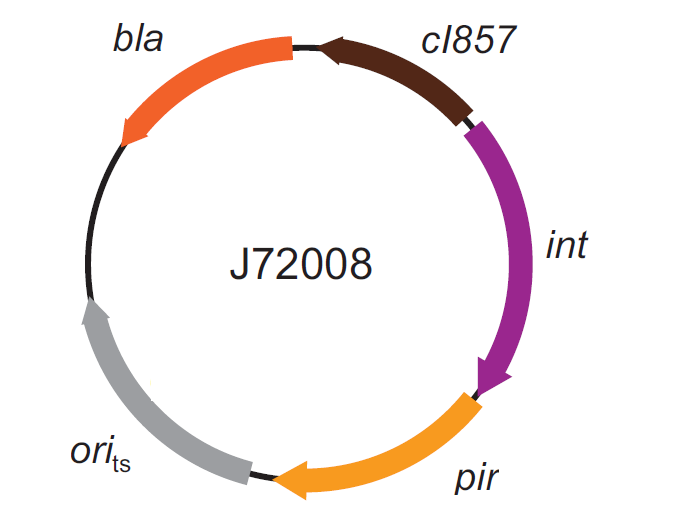
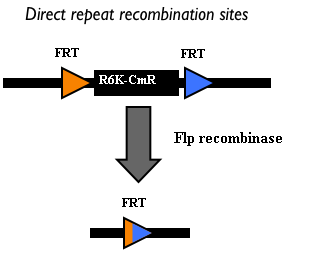
This integration method is applicable when the host strain does not have prophages in the att(Phi80) locus. TOP10 (<partinfo>BBa_V1009</partinfo>) and DH5alpha (<partinfo>BBa_V1001</partinfo>) strains have the Phi80 prophage and so their chromosome cannot be engineered with this procedure. The genomic integration of the desired BioBrick part into the attP(Phi80) locus has to be mediated by co-transforming a helper plasmid, such as the Amp-resistant <partinfo>BBa_J72008</partinfo> plasmid) which carries the IntPhi80 site-specific integrase gene under the control of a thermoinducible promoter (see Fig.5). The helper plasmid also has a heat-sensitive replication origin, whose replication can be inhibited at temperatures of 37-42°C, while a permissive temperature for this vector is 30°C. For this reason, it can be cured at high temperatures, when the integrase expression is triggered at the same time. The Phi80 integrase mediates the site-specific recombination between the attP site in the integrative vector and the attB site in the bacterial genome (for a schematic description of this process, see Fig.6 and http://partsregistry.org/Recombination). Thanks to its R6K conditional replication origin, the integrative vector cannot be replicated in common E. coli strains, so the Chloramphenicol resistant bacteria are actual integrants. In the Materials and Methods section (https://2010.igem.org/Team:UNIPV-Pavia/Project/results), a detailed protocol to target the desired BioBrick part into the Phi80 locus is reported. How to perform multiple integrations in the same genomeWhen this vector is integrated into the genome, the desired passenger should be maintained into the host, as well as the Chloramphenicol resistance marker and the R6K conditional replication origin. The CmR and the R6K can be excised from the genome by exploiting the two FRT recombination sites that flank them. The Flp recombinase protein mediates this recombination event (for a schematic description of this process, see Fig.7 and http://partsregistry.org/Recombination), so it has to be expressed by a helper plasmid, such as pCP20 (CGSC#7629). This enables the sequential integration of several parts using the same antibiotic resistance marker, which can be each time eliminated.
Detailed protocols about homologous recombination can be found here:
Integrative standard vector for yeastHere, a detailed description of the integrative vector for the yeast S. cerevisiae is reported. The structure of the designed vector, here named BBa_K300001, is shown in Fig.1. Most of its features have been inspired by the pUG6 plasmid (GenBank: AF298793.1), constructed by [Guldener U et al., 1996]. This is an integrative vector which can be used to insert the desired RFC10-compatible BioBrick parts/devices/systems into the genome of S. cerevisiae. This vector can also be specialized to target the desired integration site in the host genome. The default version of this backbone targets the Gal system of the S288C strain (<partinfo>BBa_K300979</partinfo>) through the two homologous regions <partinfo>BBa_K300986</partinfo> and <partinfo>BBa_K300987</partinfo>. The Gal system is not essential for yeast survival if the strain is grown on carbon sources other than galactose. This vector enables multiple integrations in different positions of the same genome. The usage of the KanMX dominant selection marker can avoid the usage of auxotrophic markers. In the industrial framework auxotrophies are usually deleterious for the process productivity because they affect the growth rate of cells. For this reason, this vector can be a concrete solution for the design of industrial yeast strains with novel user-defined functions.
GlossaryHR (Homologous Region) is a sequence that can recombine with the host genome.
How to propagate it before performing genome integrationThis vector can be easily propagated in E. coli thanks to the high-copy replication origin and the Ampicillin resistance selection marker, both derived from the <partinfo>pSB1A2</partinfo> vector backbone.
How to integrate a BioBrick into the yeast genome
Users can change the integration site by engineering the vector: <partinfo>BBa_K300986</partinfo> and <partinfo>BBa_K300987</partinfo> are flanked by two AvrII and two NheI respectively and for this reason the two Homologous Regions can be excided. New homologous sequences compatible with RFC10 can be digested with XbaI-SpeI and assembled because AvrII, NheI, XbaI and SpeI have compatible sticky ends. Note that this assembly is not directional and the correct orientation can be validated through sequencing with standard VF2 and VR primers.
How to perform the KanMX marker excisionThe KanMX dominant selection marker is flanked by two loxP recombination sites and for this reason it can be excided upon Cre recombinase activity. The Cre recombinase has to be expressed by a helper plasmid.
Self-cleaving affinity tags to easily purify proteins
|
 "
"