Team:UNIPV-Pavia/Calendar/June/settimana4
From 2010.igem.org
(→June, 23rd) |
(→June, 23rd) |
||
| Line 39: | Line 39: | ||
==June, 23rd== | ==June, 23rd== | ||
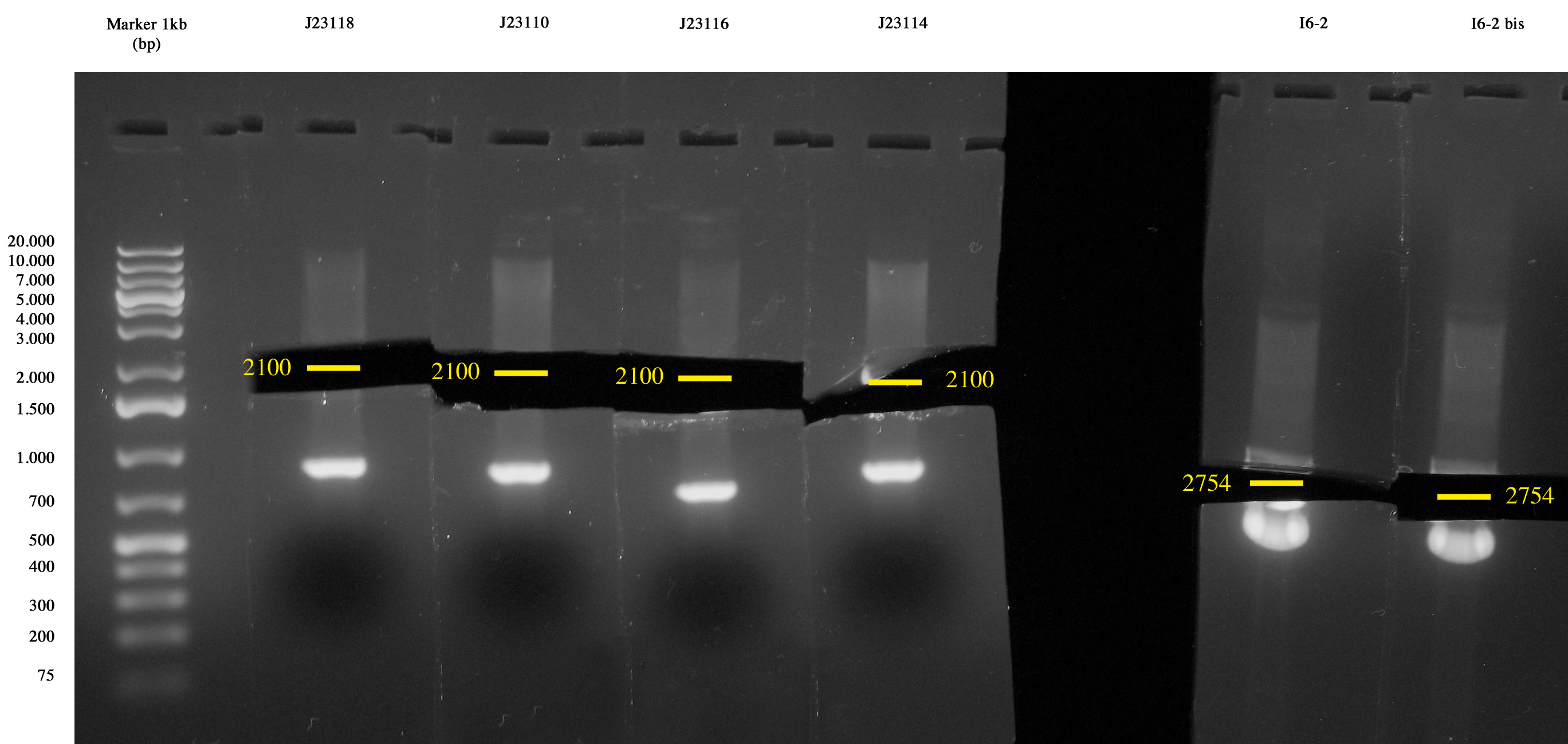
| + | Cultures incubated ON were all grown. Plasmids were extracted with MiniPrep. | ||
[[Image:Unipv_digestion_J231xx_I6.jpg|300px|thumb|center|J231xx and I6 digestion]] | [[Image:Unipv_digestion_J231xx_I6.jpg|300px|thumb|center|J231xx and I6 digestion]] | ||
| + | |||
| + | After MiniPrep, purified DNA was quantified with NanoDrop. | ||
| + | |||
| + | {| border="1" | ||
| + | | I6 || 193 ng/ul | ||
| + | |- | ||
| + | | <partinfo>BBa_J23118</partinfo> || 107,9 ng/ul | ||
| + | |- | ||
| + | | <partinfo>BBa_J23110</partinfo> || 83 ng/ul | ||
| + | |- | ||
| + | | <partinfo>BBa_J23114</partinfo> || 89,8 ng/ul | ||
| + | |- | ||
| + | | <partinfo>BBa_J23116</partinfo> || 82,7 ng/ul | ||
| + | |} | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | Digestion of: | ||
| + | |||
| + | {| border="1" | ||
| + | | ''Culture'' || ''Kind'' || ''Final reaction volume (ul) '' || ''DNA (ul)'' || ''H20 (ul)'' || ''Enzyme 1'' || ''Enzyme 2'' || ''Buffer H'' | ||
| + | |- | ||
| + | | I6 || Insert || 25 || 9,3 || 11,2 || 1 XbaI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | I6-bis || Insert || 25 ||9,3 || 11,2 || 1 XbaI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23118</partinfo> || Vector || 25 || 9,3 || 11,2 || 1 SpeI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23110</partinfo> || Vector || 25 ||12 || 8,5 || 1 SpeI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23114</partinfo> || Vector || 25 || 11,2 || 9,3 || 1 SpeI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | <partinfo>BBa_J23116</partinfo> || Vector || 25 || 12,1 || 8,4 || 1 SpeI || 1 PstI || 2,5 | ||
| + | |} | ||
| + | |||
| + | Digestions were incubated at 37°C for 3 hours, gel run and gel-extracted. | ||
| + | |||
| + | Ligations were performed ON at 16°C: | ||
| + | |||
| + | *I7: <partinfo>BBa_J23118</partinfo> (S-P)+ I6 (X-P) | ||
| + | *I8: <partinfo>BBa_J23110</partinfo> (S-P)+ I6 (X-P) | ||
| + | *I9: <partinfo>BBa_J23114</partinfo> (S-P)+ I6 (X-P) | ||
| + | *I10: <partinfo>BBa_J23116</partinfo> (S-P)+ I6 (X-P) | ||
==June, 24th== | ==June, 24th== | ||
Revision as of 08:26, 5 July 2010
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Digestion of:
Digestions were incubated at 37°C for 3 hours, gel run and gel-extracted. Ligations were performed ON at 16°C:
June, 24thJune, 25th
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
</td>
 "
"