|
JUNE
| DATE | ACTIVITY |
|---|
| June, 3th | 9° Meeting - Discussion of final and technical details about The Project. Planning of Bio-Lab activity. |
| June, 7th | 1° Bio-Lab - Susanna, Nicolò and Federica.
<partinfo>BBa_E2050</partinfo>, <partinfo>BBa_K165018</partinfo>, <partinfo>BBa_K165037</partinfo>, <partinfo>BBa_J61001</partinfo>, <partinfo>BBa_K081008</partinfo> and <partinfo>BBa_K125500</partinfo> were resuspended from Spring 2010 DNA distribution. <partinfo>BBa_P1004</partinfo> was resuspended from Spring 2009 DNA distribution. All BioBricks were transformed in E. coli DH5alpha.
Liquid LB+Amp and LB agar plates +Amp were prepared according with "protocols". |
| June, 8th | 2° Bio-Lab - Susanna, Alessandro and Chiara.
|
| June, 9th | 3° Bio-Lab - Susanna, Nicolò and Sara.
|
| June, 10th | 4° Bio-Lab - Susanna, Manuel Z. and Riccardo.
|
| June, 11th | 5° Bio-Lab - Susanna and Nicolò.
|
June, 7th
These BioBrick were resuspended in 15ul ddH20:
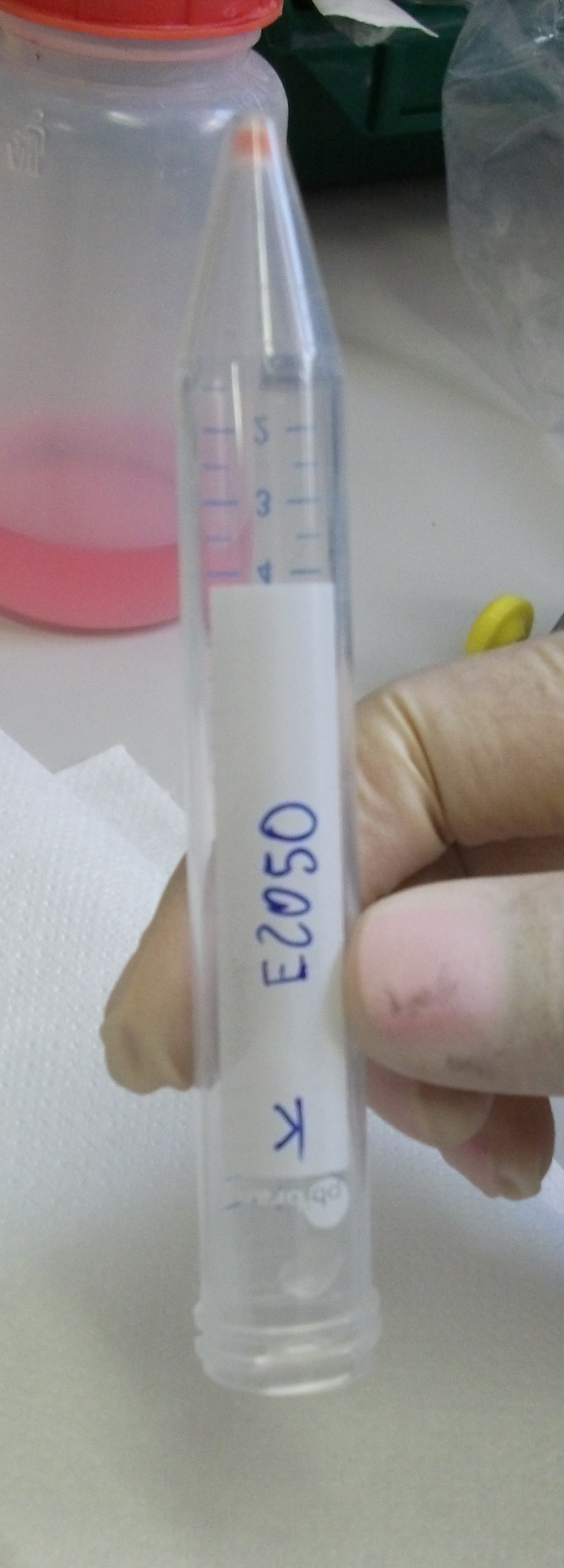
- BBa_E2050 (mOrange, 744bp) 2010 Kit Plate 2, well 13N in pSB2K3
- BBa_K165018 (ADH1 terminator, 253 bp) 2010 Kit Plate 3, well 2M, J63009 (Amp)
- BBa_K165037 (tef2 promoter, 403bp) 2010 Kit Plate 3, well 22O, pSB1AK3
- BBa_P1004 (Chloramphenicol resistence cassette, 769 bp) 2009 Kit plate 1, well 7B, pSB1A1
- BBa_J61001 (R6K origin, 406bp) 2010 Kit plate 1, well 240, pSB1A2
- BBa_K081008 (RBS-luxI, 664bp) 2010 Kit plate 2, well 10L, pSB1A2
- BBa_K125500 (GFP fusion brick, 718bp) 2010 Kit plate 3, well 2P, pSB1A2
1,5 ul of each culture was transformed in 100 ul of home-made competent E. coli DH5alpha.
Tranformants were all plated on LB agar plates added with Ampicillin, except for BBa_E2050 (Kanamycin).
500 ml LB+Amp was prepared and 21 LB agar plates + Amp were prepared (500 ml).
June, 8th
All plates grown overnight at 37°C show colonies!
In particular, <partinfo>BBa_E2050</partinfo> (gorwn on LB+Kan), <partinfo>BBa_K125500</partinfo> (gorwn on LB+Amp), <partinfo>BBa_K081008</partinfo> (gorwn on LB+Amp) and <partinfo>BBa_K165018</partinfo> (gorwn on LB+Amp) showed big colonies, well separated o the plate.
<partinfo>BBa_P1004</partinfo> (gorwn on LB+Amp) and <partinfo>BBa_J61001</partinfo> (gorwn on LB+Amp) showed many colonies, with big colonies surrounded by small colonies.
<partinfo>BBa_K165037</partinfo> (gorwn on LB+Amp) showed only 11 big colonies.
A colony was peaked for each plate and inoculated in 1ml LB+antibiotic.
| <partinfo>BBa_E2050</partinfo> | 1ml LB+Kan
|
| <partinfo>BBa_K165037</partinfo> | 1ml LB+Amp
|
| <partinfo>BBa_P1004</partinfo> | 1ml LB+Amp
|
| <partinfo>BBa_K125500</partinfo> | 1ml LB+Amp
|
| <partinfo>BBa_K081008</partinfo> | 1ml LB+Amp
|
| <partinfo>BBa_J61001</partinfo> | 1ml LB+Amp
|
| <partinfo>BBa_K165018</partinfo> | 1ml LB+Amp
|
|
|

|
<partinfo>BBa_K165037</partinfo> in pSB1AK3 plasmid was inocultaed both in 1ml LB+Amp and in 1ml LB+Kan for a phenotipic assay.
Cultures were grown for 8 hours at 37°C 220 rpm.
After this time, cultures were all grown and in saturation phase.
Glycerol stocks were prepared for each culture (250ul saturated culture + 750 ul glycerol 80%) and stored at -80°C).
The left culture was re-filled to 5ml with LB+antibiotic and grown overnight at 37°C 200 rpm to be miniprepped tomorrow.
June, 9th
June, 10th
June, 11th
|
|