Team:UNIPV-Pavia/Calendar/August/settimana1
From 2010.igem.org
m (→August, 3rd) |
m (→August, 5th) |
||
| (51 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
<td valign="top"> | <td valign="top"> | ||
<table border="0" align="center" width="100%"><tr><td align="justify" valign="top" style="padding:20px"> | <table border="0" align="center" width="100%"><tr><td align="justify" valign="top" style="padding:20px"> | ||
| + | <table class="menu" border="0" width="100%"> | ||
| + | <tr> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/August/settimana1|Week 1]] | ||
| + | </td> | ||
| + | <td align="center" style="padding:0; height:20px"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/August/settimana2|Week 2]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/August/settimana3|Week 3]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/August/settimana4|Week 4]] | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | |||
<html><p align="center"><font size="4"><b>AUGUST: WEEK 1</b></font></p></html><hr><br> | <html><p align="center"><font size="4"><b>AUGUST: WEEK 1</b></font></p></html><hr><br> | ||
| + | <html><a name="indice"/></html> | ||
==August, 2nd== | ==August, 2nd== | ||
Miniprep and quantification with Nanodrop of: | Miniprep and quantification with Nanodrop of: | ||
| Line 32: | Line 51: | ||
{|border="1" align="center" | {|border="1" align="center" | ||
|- | |- | ||
| - | |'''Part''' || '''Strain''' | + | |'''Part''' || '''Strain''' || '''Culture name''' |
|- | |- | ||
| - | |rowspan="2"| pAH123 || MC1061 | + | |rowspan="2"| pAH123 || MC1061 || MC123 |
|- | |- | ||
| - | |MG1655 | + | |MG1655 || MG123 |
|- | |- | ||
| - | |rowspan="2"| <partinfo> | + | |rowspan="2"| <partinfo>BBa_J72008</partinfo> || MC1061 || MC008 |
|- | |- | ||
| - | |MG1655 | + | |MG1655 || MG008 |
|- | |- | ||
| - | |<partinfo>BBa_R0062</partinfo> ||rowspan="2"| DH5-alpha | + | |<partinfo>BBa_R0062</partinfo> ||rowspan="2"| DH5-alpha || rowspan="2"| |
|- | |- | ||
|<partinfo>BBa_K081009</partinfo> | |<partinfo>BBa_K081009</partinfo> | ||
| Line 55: | Line 74: | ||
|pAH123 ||rowspan="2"| Amp 50 ug/ml ||rowspan="2"| 30°C | |pAH123 ||rowspan="2"| Amp 50 ug/ml ||rowspan="2"| 30°C | ||
|- | |- | ||
| - | |<partinfo> | + | |<partinfo>BBa_J72008</partinfo> |
|- | |- | ||
|<partinfo>BBa_R0062</partinfo> || rowspan="2"| Amp 100 ug/ml ||rowspan="2"| 37°C | |<partinfo>BBa_R0062</partinfo> || rowspan="2"| Amp 100 ug/ml ||rowspan="2"| 37°C | ||
| Line 89: | Line 108: | ||
*Blank (Nothing) | *Blank (Nothing) | ||
| - | Gel run of amplified DNA showed for every sample the expected distance between primers of a strain with nothing integrated in attPhi80 site (~ | + | Gel run of amplified DNA showed for every sample the expected distance between primers of a strain with nothing integrated in attPhi80 site (~570 bp), but unfortunately we forgot to take a picture of the gel ;( |
| + | |||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==August, 4th== | ==August, 4th== | ||
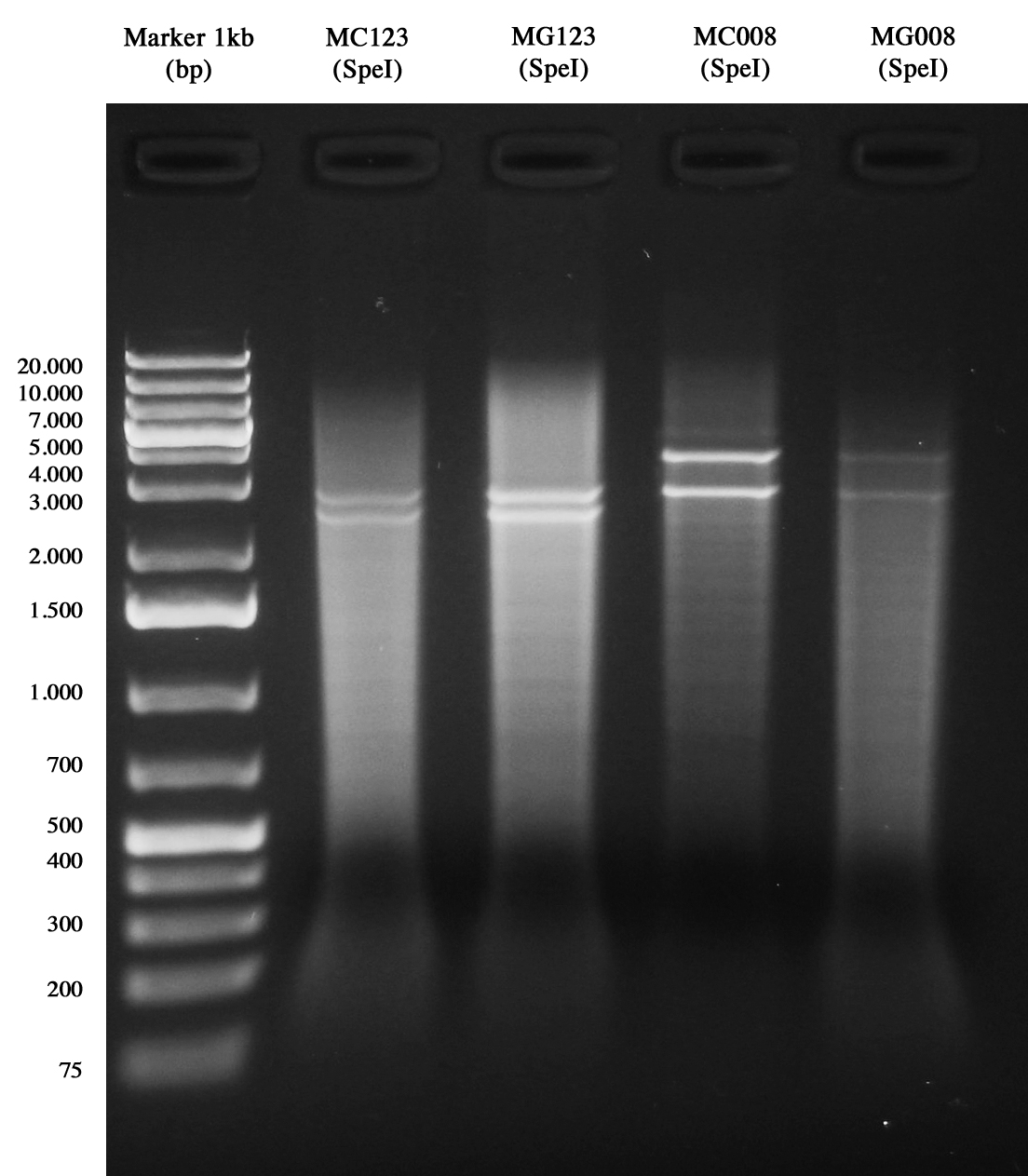
| + | Glycerol stocks for MC1061 and MG1655 strains transformed with pAH123 or <partinfo>BBa_J72008</partinfo> helper plasmids. | ||
| + | |||
| + | 2 ul of MC1061 bacteria were transferred into 5 ml LB+Amp 50 ug/ml and grown and shaken at 30°C over-day and over-night for re-competentization of the following day. | ||
| + | |||
| + | 200 ul of MG1655 cultures were transferred into 100 ml LB+Amp 50 ug/ml and grown and shaken at 30°C for re-competentization of today. | ||
| + | |||
| + | All cultures were miniprepped to check again the presence of helper plasmids. | ||
| + | |||
| + | Samples were digested with SpeI (it cuts twice) for 3 hours and gel run: | ||
| + | *pAH123 digested: 3580 and 2755 bp | ||
| + | *<partinfo>BBa_J72008</partinfo> digested: 2755 and 2437 bp | ||
| + | |||
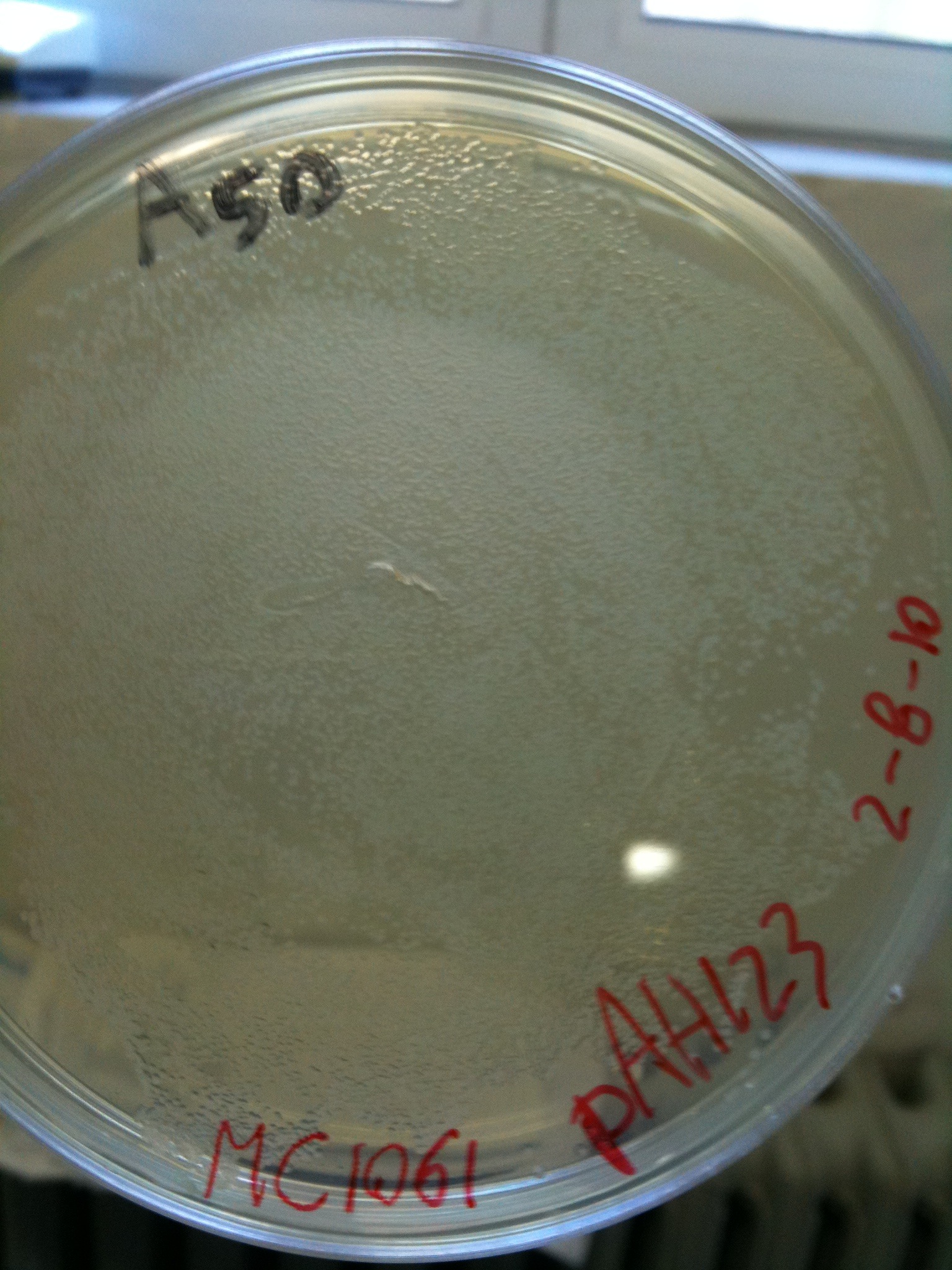
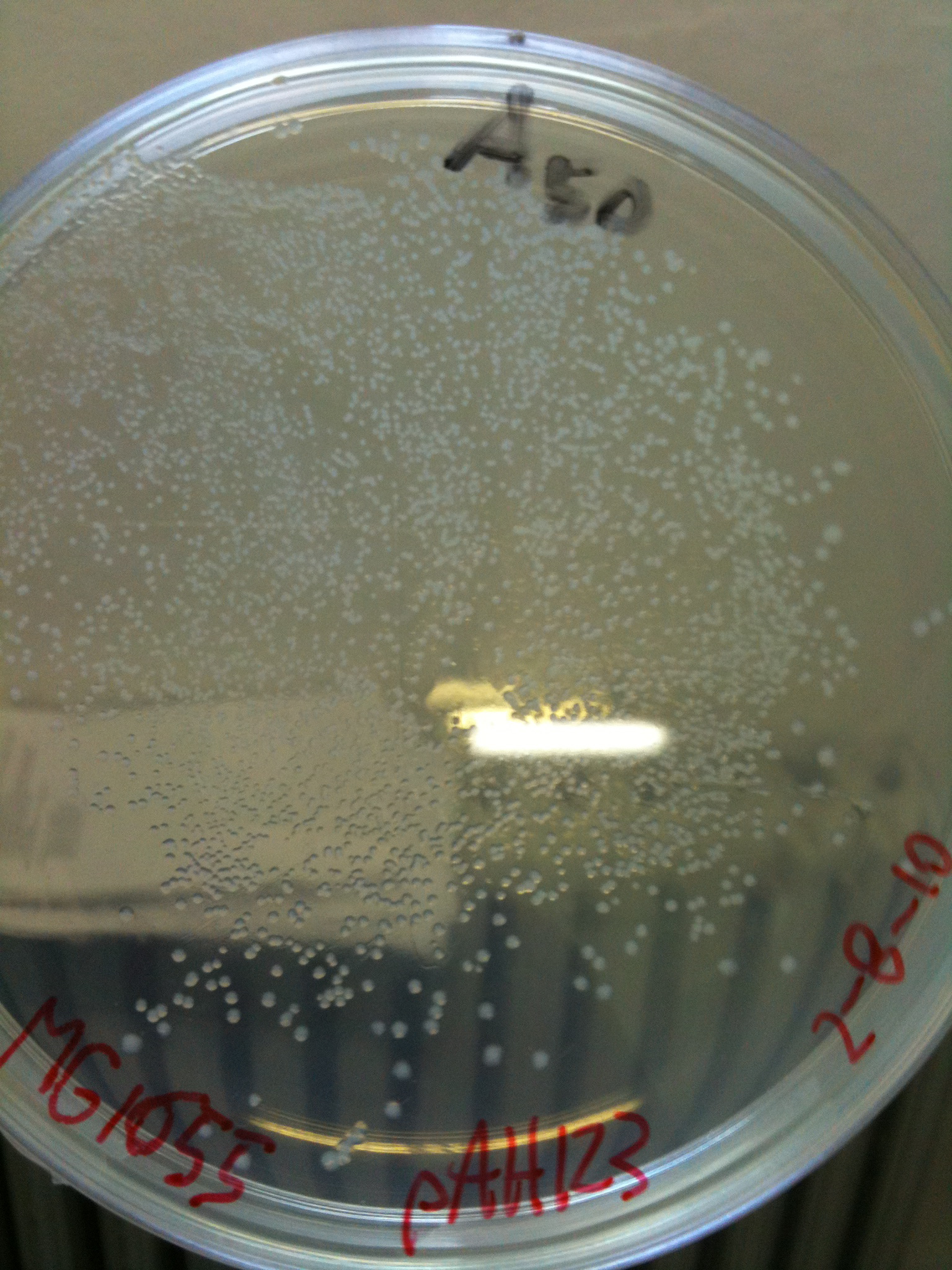
| + | [[Image:UNIPV10_MG1655_MC1061_helper_screening.jpg|thumb|200px|center|pAH123 and <partinfo>BBa_J72008</partinfo> screening transformed into MG1655 and MC1061]] | ||
| + | |||
| + | Samples are positive (right lengths) but unfortunately we got a bad gel run (smearings) so this time we decided to pick two single colonies from each of the plates made on August, 3rd and to inoculate them into 5 ml LB+Amp 50 ug/ml. A total of eight falcon tubes was incubated ON at 30°C, 220 rpm. | ||
| + | |||
| + | We planned to screen them (to check the presence of helper plasmids again) and to re-competentize only the positive ones. | ||
| + | |||
| + | ---- | ||
| + | |||
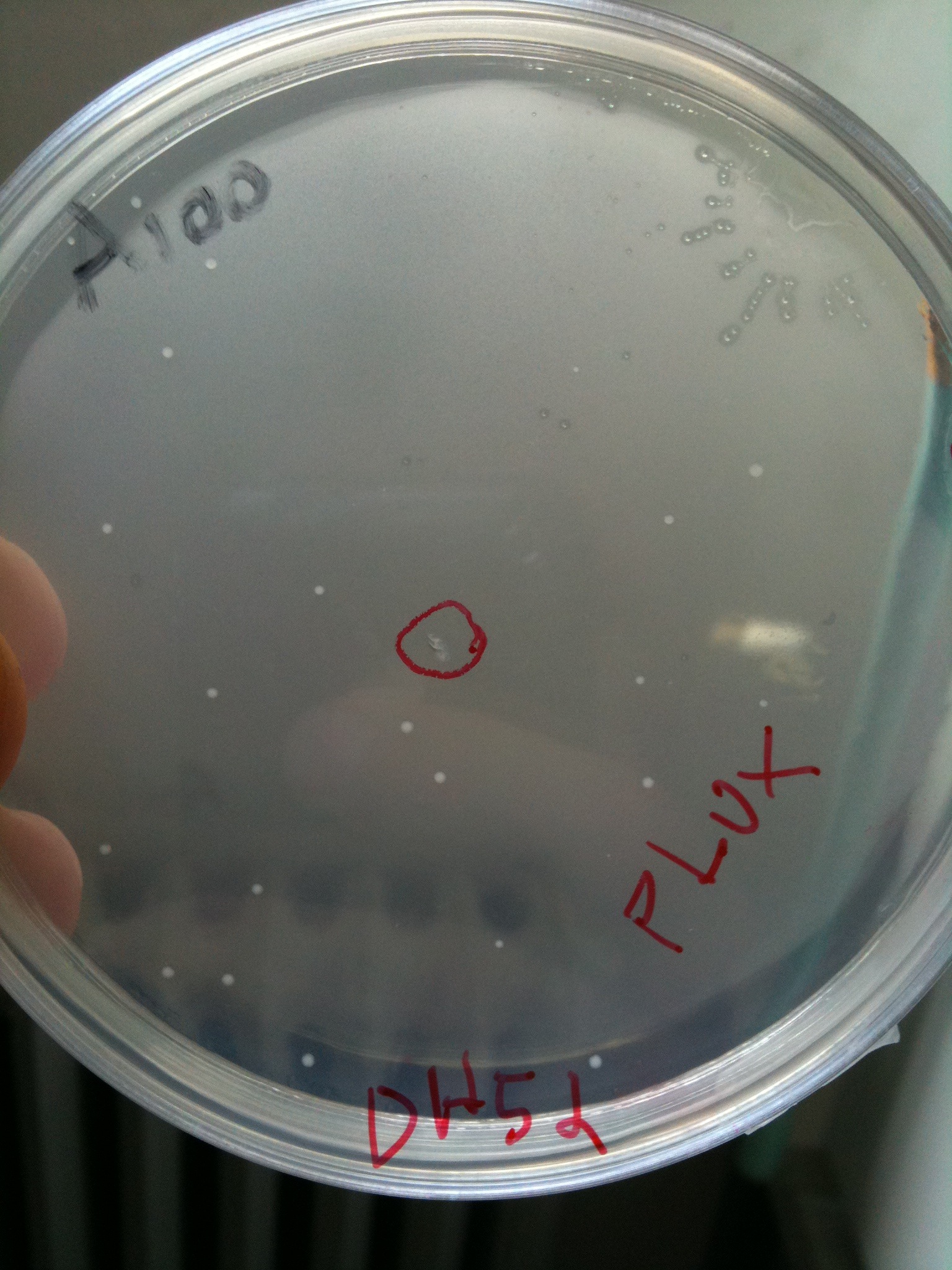
| + | [[Image:UNIPV10_plux.jpg|thumb|200px|center|Autoinducibles]] | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==August, 5th== | ==August, 5th== | ||
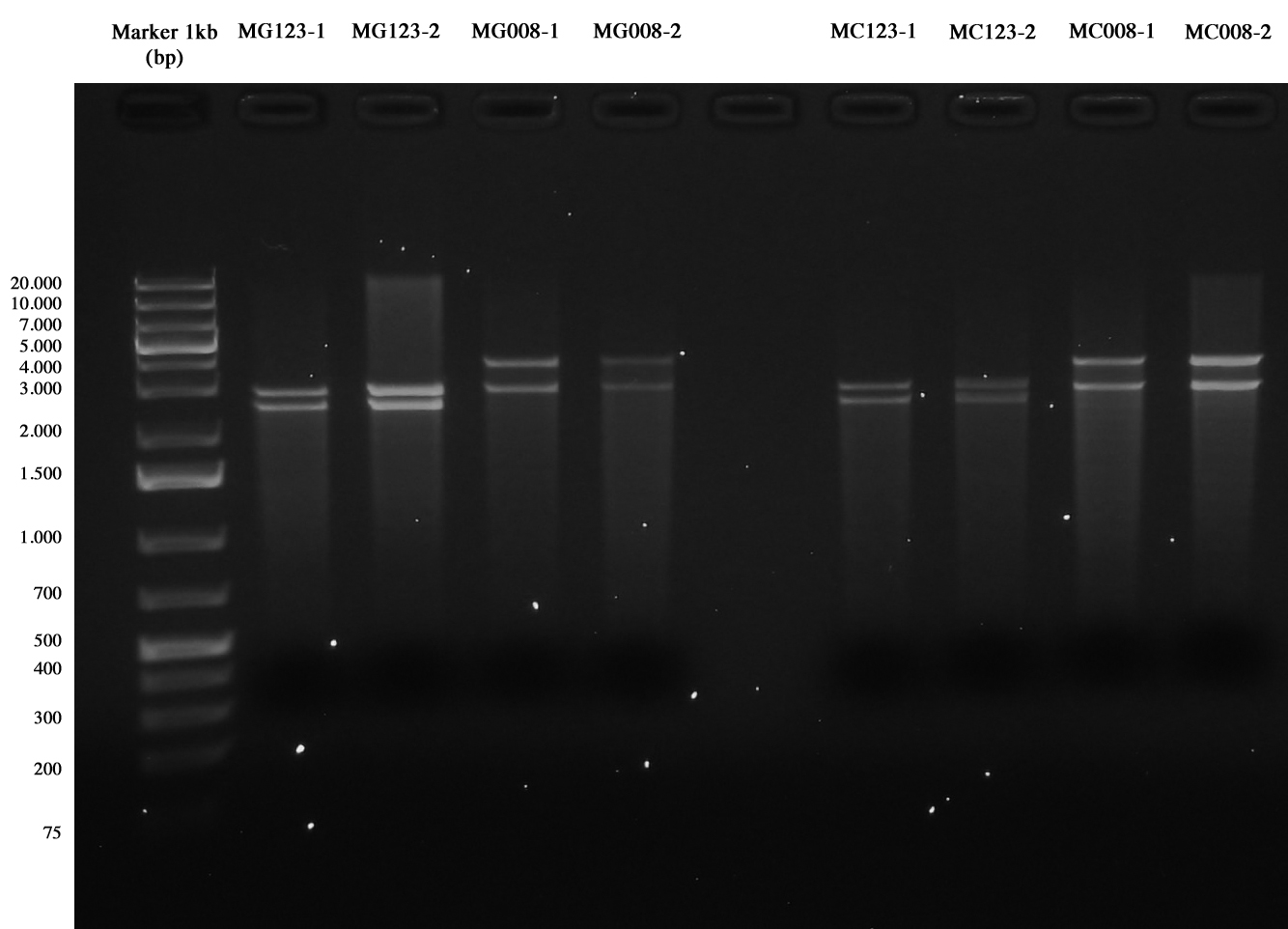
| + | Glycerol stock and miniprep of MG1655 and MC1061 cultures incubated for 19 hours at 30°C, 220 rpm. | ||
| + | Miniprep was quantified as follows: | ||
| + | *MG123-1: 27 ng/ul | ||
| + | *MG123-2: 40,2 ng/ul | ||
| + | *MG008-1: 20,5 ng/ul | ||
| + | *MG008-2: 26,5 ng/ul | ||
| + | *MC123-1: 21,4 ng/ul | ||
| + | *MC123-2: 17,4 ng/ul | ||
| + | *MC008-1: 33,3 ng/ul | ||
| + | *MC008-2: 38,1 ng/ul | ||
| + | |||
| + | DIgestion for 1 hour with SpeI. | ||
| + | |||
| + | Gel run on medium agarose gel. | ||
| + | |||
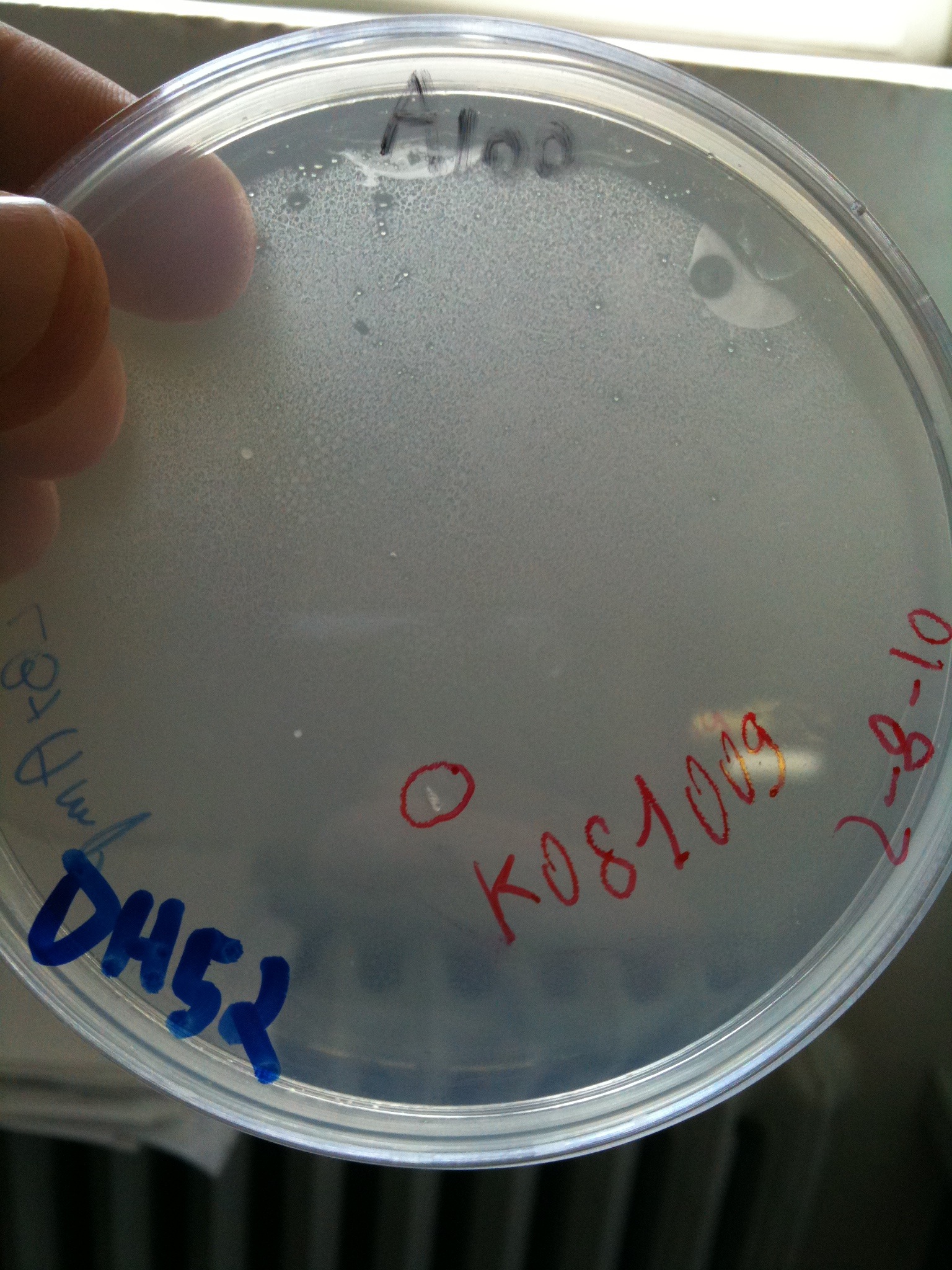
| + | [[Image:UNIPV10_transformed_helper_screening.jpg|thumb|200px|center|pAH123 and <partinfo>BBa_J72008</partinfo> screening (miniprepped from MG1655 and MC1061)]] | ||
| + | |||
| + | This time gel run succeeded, we chose samples | ||
| + | *MG123-1 | ||
| + | *MG008-1 | ||
| + | *MC123-1 | ||
| + | *MC008-1 | ||
| + | to be re-competentizied. So they were inoculated into 5 ml LB+Amp 50 ug/ml and grown and shaken ON at 30°C. | ||
| + | ---- | ||
| + | Sequencing for I20 and I21 arrived from BMR, but all samples were wrong; sites X and S of the vector paired and nothing could ligate. So we started a new ligation cycle dephosphorylating the previously gel-extracted vector <partinfo>pSB1A3</partinfo>. | ||
| + | New ligations: | ||
| + | *I20-new: Pha-10S-1 (X-S) + <partinfo>pSB1A3</partinfo> (X-S, dephosphorylated) | ||
| + | *I21-new: Pha-SS-1 (X-S) + <partinfo>pSB1A3</partinfo> (X-S, dephosphorylated) | ||
| + | and their negative control: | ||
| + | *C-: <partinfo>pSB1A3</partinfo> (X-S, dephosphorylated) | ||
| + | |||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==August, 6th== | ==August, 6th== | ||
| + | Resuspension of linker <partinfo>BBa_K105012</partinfo> from iGEM 2010 Distribution Kit. | ||
| + | |||
| + | Transformation of ligations and resuspended DNA | ||
| + | *I20-new | ||
| + | *I21-new | ||
| + | *C- | ||
| + | *<partinfo>BBa_K105012</partinfo> | ||
| + | into 100ul ''E. coli DH5-alpha''. | ||
| + | |||
| + | They were plated on LB+Amp 100ug/ml agar plates | ||
| + | |||
| + | ---- | ||
| + | Competentization of colonies selected the previous day: | ||
| + | *MG123 (without '-1' from now on) | ||
| + | *MG008 | ||
| + | *MC123 | ||
| + | *MC008 | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==August, 7th== | ==August, 7th== | ||
| + | Plates of transformed cells were stored at +4°C. | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| + | |||
| + | |||
| - | == | + | <!-- table previous next week --> |
| + | <br><br> | ||
| + | <table border="0" width="100%" class="menu"> | ||
| + | <tr> | ||
| + | <td align="left">[[Team:UNIPV-Pavia/Calendar/July/settimana5| Previous week]]</td> | ||
| + | <td align="right">[[Team:UNIPV-Pavia/Calendar/August/settimana2| Next week]]</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <!-- fine table previous next week --> | ||
| + | </td> | ||
| + | <td width="15%" align="right" valign="top"> | ||
| - | + | {{UNIPV-Pavia/menu_mesi}} | |
| - | + | </td> | |
| - | + | </tr> | |
| - | + | ||
| - | </td | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</table> | </table> | ||
Latest revision as of 16:58, 24 October 2010
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"