Team:TzuChiU Formosa/Notebook
From 2010.igem.org
Vanessayang (Talk | contribs) (→Team 2: Poseidon - Save the World) |
|||
| Line 36: | Line 36: | ||
* Note: | * Note: | ||
| - | # For all of our construct assembly ,we designed XbaⅠrestriction site at | + | # For all of our construct assembly ,we designed XbaⅠrestriction site at 5’ end and SpeⅠat 3’ end.<br> |
| - | # We design the plus strain & minus strain of RBS (BBa_B0034) , Biobrick cloning site prefix , and suffix, then we can anneal complementary single strain into double strain fragments. There is an additional adenine at | + | # We design the plus strain & minus strain of RBS (BBa_B0034), Biobrick cloning site prefix, and suffix, then we can anneal complementary single strain into double strain fragments. There is an additional adenine at 3' end of each single strain, it is designed for ligation on pGEM®-T Easy Vector (Promega).<br> |
| Line 52: | Line 52: | ||
# pipette mix DNA<br> | # pipette mix DNA<br> | ||
# transfer DNA solution to new eppendorf<br> | # transfer DNA solution to new eppendorf<br> | ||
| - | # storage at - | + | # storage at -20℃<br> |
* Transform GFP generator (BBa_E0240) into XL-10 Gold competent cell: | * Transform GFP generator (BBa_E0240) into XL-10 Gold competent cell: | ||
| - | # Take out XL-10 Gold competent cell from - | + | # Take out XL-10 Gold competent cell from -80℃ refrigerator; put on ice<br> |
# Add 2 μl GFP generator DNA solution into competent cell<br> | # Add 2 μl GFP generator DNA solution into competent cell<br> | ||
# Place on ice, 30 min<br> | # Place on ice, 30 min<br> | ||
| - | # Heat shock, | + | # Heat shock, 42℃, 90 sec<br> |
# Chill on ice, 5 min<br> | # Chill on ice, 5 min<br> | ||
# Add 800 μl LB medium (without antibiotics)<br> | # Add 800 μl LB medium (without antibiotics)<br> | ||
| - | # Incubate at | + | # Incubate at 37℃, 1 hr<br> |
| - | # bacteria solution centrifuge 3000 rpm, | + | # bacteria solution centrifuge 3000 rpm, 5 min<br> |
# Remove 700 μl supernatant<br> | # Remove 700 μl supernatant<br> | ||
| - | # Smear 100 μl Ampicilin (50 mg /ml) over LB agar plate<br> | + | # Smear 100 μl Ampicilin (50 mg/ml) over LB agar plate<br> |
# Mix bacteria solution; Smear 100 μl over LB agar plate<br> | # Mix bacteria solution; Smear 100 μl over LB agar plate<br> | ||
| - | # Incubate at | + | # Incubate at 37℃, overnight<br> |
Day 4: Sep 4 ,2010 | Day 4: Sep 4 ,2010 | ||
| + | |||
* Mini-prep : GFP generator (BBa_E0240) transformants | * Mini-prep : GFP generator (BBa_E0240) transformants | ||
# Pick 4 colonies from GFP generator transformants<br> | # Pick 4 colonies from GFP generator transformants<br> | ||
| - | # Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg /l)); 37 overnight<br> | + | # Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg/l)); 37 overnight<br> |
Day 5: Sep- 5 ,2010 | Day 5: Sep- 5 ,2010 | ||
| + | |||
* Plasmid extraction (Homemade) : GFP generator (BBa_E0240) transformants | * Plasmid extraction (Homemade) : GFP generator (BBa_E0240) transformants | ||
# Centrifuge 3000 rpm, 10 min; remove supernatant<br> | # Centrifuge 3000 rpm, 10 min; remove supernatant<br> | ||
| Line 82: | Line 84: | ||
# Centrifuge 13000 rpm, 10 min<br> | # Centrifuge 13000 rpm, 10 min<br> | ||
# Transfer supernatant to new 1.5 ml microcentrifuge tube<br> | # Transfer supernatant to new 1.5 ml microcentrifuge tube<br> | ||
| - | # Add 1 ml 100 % EtOH<br> | + | # Add 1 ml 100% EtOH<br> |
# Centrifuge 13000 rpm, 15 min<br> | # Centrifuge 13000 rpm, 15 min<br> | ||
# Remove supernatant<br> | # Remove supernatant<br> | ||
| - | # Wash pellet 1 ml 75 % EtOH; remove supernatant; air-dry DNA pellet<br> | + | # Wash pellet 1 ml 75% EtOH; remove supernatant; air-dry DNA pellet<br> |
# Dissolve DNA pellet with 50 μl ddH2O<br> | # Dissolve DNA pellet with 50 μl ddH2O<br> | ||
* GFP generator (BBa_E0240) enzyme digestion (check) | * GFP generator (BBa_E0240) enzyme digestion (check) | ||
| Line 93: | Line 95: | ||
# 0.3 μl BSA<br> | # 0.3 μl BSA<br> | ||
# 10 μl DNA<br> | # 10 μl DNA<br> | ||
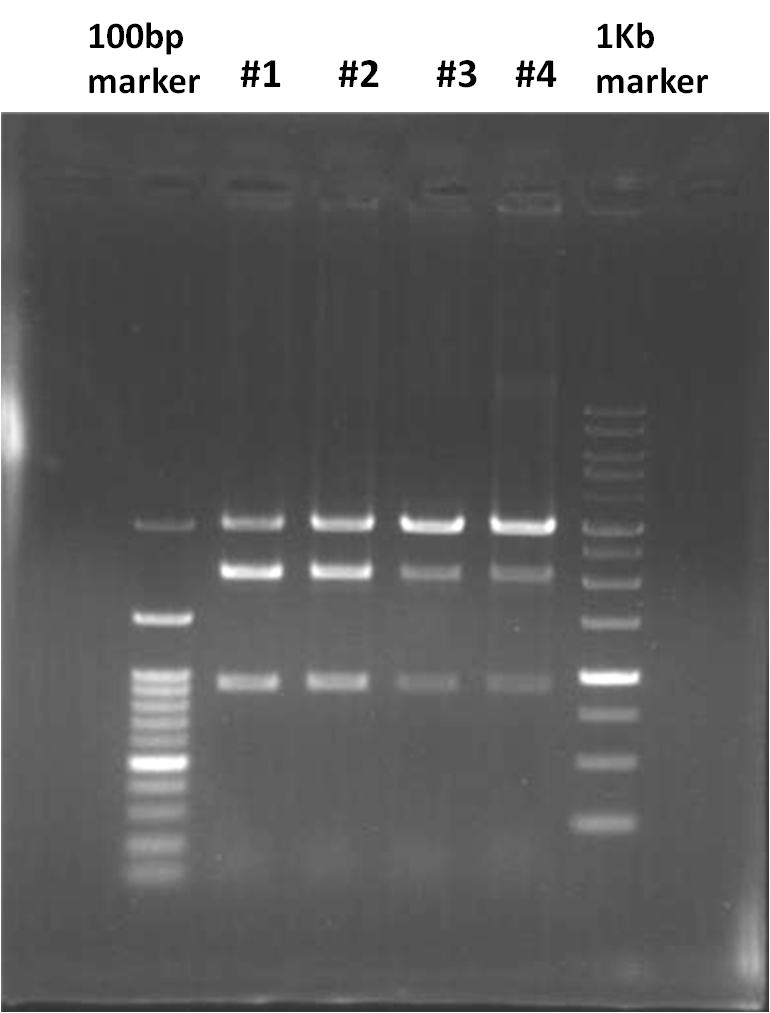
| - | # | + | # Incubate at 37℃, 3 hr <br> |
[[Image:2010.09.05.png|300px|2010.09.05]] | [[Image:2010.09.05.png|300px|2010.09.05]] | ||
| Line 101: | Line 103: | ||
* Primers have arrived!! | * Primers have arrived!! | ||
| - | # Dilute primers to 100μM /μl; Take out 10 μl, add 90 μl ddH2O (dilute to 10μM /μl)<br> | + | # Dilute primers to 100μM/μl; Take out 10 μl, add 90 μl ddH2O (dilute to 10μM/μl)<br> |
| Line 111: | Line 113: | ||
# 1 μl template<br> | # 1 μl template<br> | ||
# 9.5 μl ddH2O<br> | # 9.5 μl ddH2O<br> | ||
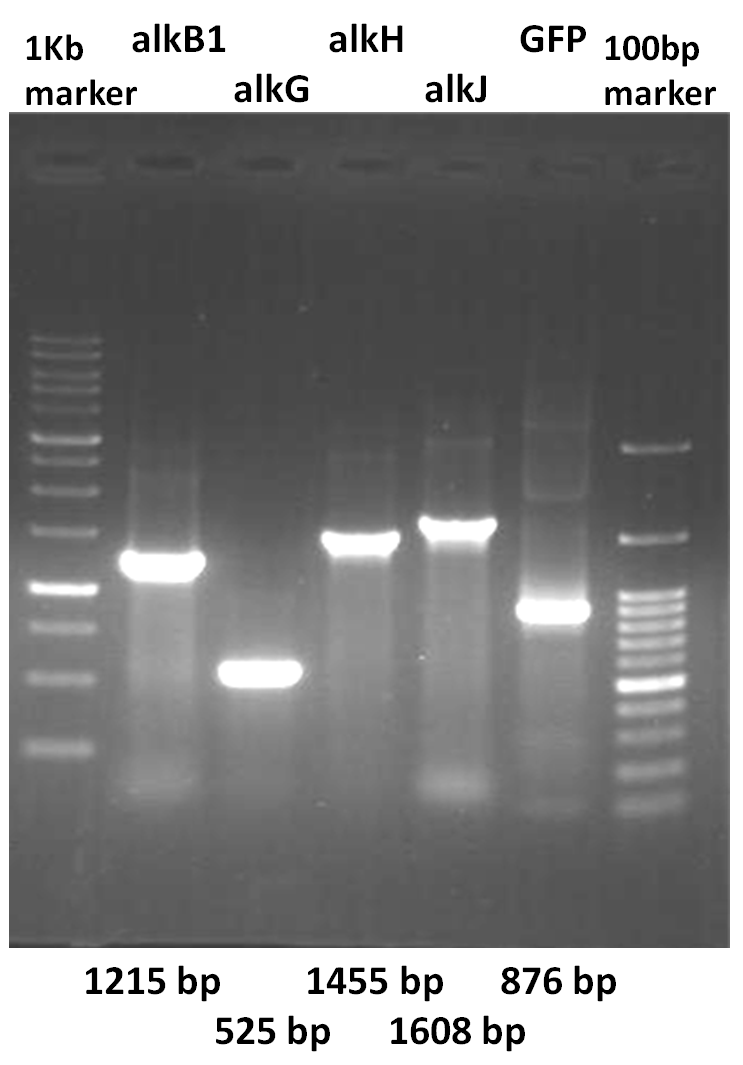
| - | # cycle 95/2 min; 30x(95/1 min;56/2 min;72/1.5 min); 72/7 min<br> | + | # cycle 95/2 min; 30x(95/1 min; 56/2 min; 72/1.5 min); 72/7 min<br> |
[[Image:2010.09.07.png|300px|2010.09.07]] | [[Image:2010.09.07.png|300px|2010.09.07]] | ||
* Gel extraction: alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes | * Gel extraction: alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes | ||
| - | + | - use Gel/PCR DNA Fragments Extraction Kit (Geneaid)<br> | |
# Loading the PCR products into agarose gel; run gel with 1X TAE buffer<br> | # Loading the PCR products into agarose gel; run gel with 1X TAE buffer<br> | ||
| - | # Excise the agarose gel slice containing relevant DNA | + | # Excise the agarose gel slice containing relevant DNA fragments <br> |
| - | + | ||
# Transfer gel slice to a 1.5 ml microcentrifuge tube<br> | # Transfer gel slice to a 1.5 ml microcentrifuge tube<br> | ||
| + | # Add 500 μl DF buffer; incubate at 56℃ until gel slice has been completely dissolved<br> | ||
| + | # Transfer 800 μl gel mixture to DF column<br> | ||
| + | # Centrifuge 13000 rpm, 1 min<br> | ||
| + | # Discard the flow-through and place the DF column back<br> | ||
| + | # Add 400 μl W1 buffer; Centrifuge 13000 rpm, 1 min; Discard the flow-through<br> | ||
| + | # Add 600 μl wash buffer; Centrifuge 13000 rpm, 1 min; Discard the flow-through<br> | ||
| + | # Centrifuge 13000 rpm, 5 min; air-dry<br> | ||
| + | # Add 50 μl ddH2O; Centrifuge 13000 rpm,1 min<br> | ||
| + | # Storage at –20℃<br> | ||
* TA ligation: alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes | * TA ligation: alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes | ||
| - | + | - use pGEM®-T Easy Vector (Promega)<br> | |
| + | # 1μl pGEM®-T Easy Vector<br> | ||
| + | # 5μl 2X rapid ligation buffer<br> | ||
| + | # 1μl T4 DNA ligase<br> | ||
| + | # 3μl insert<br> | ||
| + | # 4℃, overnight<br> | ||
| Line 128: | Line 143: | ||
* Transformation | * Transformation | ||
| - | + | - transform alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) TA ligation products into XL-10 Gold competent cell<br> | |
Day 9: Sep 9 ,2010 | Day 9: Sep 9 ,2010 | ||
| + | |||
* Annealing | * Annealing | ||
| - | + | - anneal complementary single strain of RBS (BBa_B0034), Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) into double strain fragments<br> | |
| - | # dilute oligo primers to 10 μM /μl<br> | + | # dilute oligo primers to 10 μM/μl<br> |
# add 12.5 μl plus strain<br> | # add 12.5 μl plus strain<br> | ||
# add 12.5 μl minus strain<br> | # add 12.5 μl minus strain<br> | ||
| - | # Heat to | + | # Heat to 95℃; cool down to room temperature slowly <br> |
* TA ligation | * TA ligation | ||
| - | + | - Ligate RBS (BBa_B0034) , Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) on pGEM®-T Easy Vector (Promega)<br> | |
| + | # 1μl pGEM®-T Easy Vector<br> | ||
| + | # 5μl 2X rapid ligation buffer<br> | ||
| + | # 1μl T4 DNA ligase<br> | ||
| + | # 3μl insert<br> | ||
| + | # 4℃, overnight<br> | ||
* Mini-prep | * Mini-prep | ||
| - | + | - Pick alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) transformants (ligate on pGEM-T easy vector)<br> | |
# Pick 4 colonies from alkB1, alkG, alkH, alkJ and GFP generator transformants<br> | # Pick 4 colonies from alkB1, alkG, alkH, alkJ and GFP generator transformants<br> | ||
| - | # Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg /l)); | + | # Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg/l)); 37℃, overnight<br> |
| + | |||
| + | |||
| + | Day10: Sep 10 ,2010 | ||
| + | |||
| + | * Plasmid extraction (Homemade) : alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) transformants (ligate on pGEM-T easy vector) | ||
| + | * Enzyme digestion (check) | ||
| + | # 6 μl RNase <br> | ||
| + | # 3 μl NEB buffer 4<br> | ||
| + | # 0.5 μl XbaⅠ<br> | ||
| + | # 0.5 μl SpeⅠ<br> | ||
| + | # 10 μl plasmid DNA<br> | ||
| + | # Incubate at 37℃, 3 hr<br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | * Transformation | ||
| + | - transform RBS (BBa_B0034), Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) into XL-10 Gold competent cell<br> | ||
| + | |||
| + | |||
| + | Day11: Sep 11 ,2010 | ||
| + | |||
| + | * Mini-prep | ||
| + | - Pick RBS (BBa_B0034), Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) transformants (ligate on pGEM-T easy vector)<br> | ||
| + | # Pick 4 colonies from RBS , Biobrick cloning site prefix and Biobrick cloning site suffix transformants<br> | ||
| + | # Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg/l)); 37℃, overnight<br> | ||
| + | - alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) (have been checked in correct size and ligate on pGEM-T easy vector) mini-prep for plasmid extraction (use plasmid extraction Kit)<br> | ||
| + | # 5 μl bacterial stock<br> | ||
| + | # 10 ml LB (with Ampicilin (50mg/l))<br> | ||
| + | # Incubate at 37℃, overnight<br> | ||
Revision as of 12:52, 12 September 2010
Notebook
You should make use of the calendar feature on the wiki and start a lab notebook. This may be looked at by the judges to see how your work progressed throughout the summer. It is a very useful organizational tool as well.
Team 2: Poseidon - Save the World
Day 1: Sep 1 ,2010
- Primer design:
|
- Note:
- For all of our construct assembly ,we designed XbaⅠrestriction site at 5’ end and SpeⅠat 3’ end.
- We design the plus strain & minus strain of RBS (BBa_B0034), Biobrick cloning site prefix, and suffix, then we can anneal complementary single strain into double strain fragments. There is an additional adenine at 3' end of each single strain, it is designed for ligation on pGEM®-T Easy Vector (Promega).
Day 2: Sep 2 ,2010
- Order primers
Day 3: Sep 3 ,2010
- Cyanobacteria ATCC 51142 has arrived!!
- Take out GFP generator (BBa_E0240) from biobrick kit plate 1(site:12M):
- dilute DNA with 10 μl ddH2O
- pipette mix DNA
- transfer DNA solution to new eppendorf
- storage at -20℃
- Transform GFP generator (BBa_E0240) into XL-10 Gold competent cell:
- Take out XL-10 Gold competent cell from -80℃ refrigerator; put on ice
- Add 2 μl GFP generator DNA solution into competent cell
- Place on ice, 30 min
- Heat shock, 42℃, 90 sec
- Chill on ice, 5 min
- Add 800 μl LB medium (without antibiotics)
- Incubate at 37℃, 1 hr
- bacteria solution centrifuge 3000 rpm, 5 min
- Remove 700 μl supernatant
- Smear 100 μl Ampicilin (50 mg/ml) over LB agar plate
- Mix bacteria solution; Smear 100 μl over LB agar plate
- Incubate at 37℃, overnight
Day 4: Sep 4 ,2010
- Mini-prep : GFP generator (BBa_E0240) transformants
- Pick 4 colonies from GFP generator transformants
- Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg/l)); 37 overnight
Day 5: Sep- 5 ,2010
- Plasmid extraction (Homemade) : GFP generator (BBa_E0240) transformants
- Centrifuge 3000 rpm, 10 min; remove supernatant
- Resuspend bacteria with 100 μl Solution Ⅰ
- Add 200 μl Solution Ⅱ; gently mix
- Add 150 μl Solution Ⅲ; gently mix
- Centrifuge 13000 rpm, 10 min
- Transfer supernatant to new 1.5 ml microcentrifuge tube
- Add 1 ml 100% EtOH
- Centrifuge 13000 rpm, 15 min
- Remove supernatant
- Wash pellet 1 ml 75% EtOH; remove supernatant; air-dry DNA pellet
- Dissolve DNA pellet with 50 μl ddH2O
- GFP generator (BBa_E0240) enzyme digestion (check)
- 6 μl RNase
- 0.5 μl EoRⅠ
- 0.5 μl PstⅠ
- 0.3 μl BSA
- 10 μl DNA
- Incubate at 37℃, 3 hr
Day 6: Sep 6 ,2010
- Primers have arrived!!
- Dilute primers to 100μM/μl; Take out 10 μl, add 90 μl ddH2O (dilute to 10μM/μl)
Day 7: Sep 7 ,2010
- PCR (total volume: 25 μl ): alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes
- 12.5 μl Green master mix (Promega)
- 1 μl each primer
- 1 μl template
- 9.5 μl ddH2O
- cycle 95/2 min; 30x(95/1 min; 56/2 min; 72/1.5 min); 72/7 min
- Gel extraction: alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes
- use Gel/PCR DNA Fragments Extraction Kit (Geneaid)
- Loading the PCR products into agarose gel; run gel with 1X TAE buffer
- Excise the agarose gel slice containing relevant DNA fragments
- Transfer gel slice to a 1.5 ml microcentrifuge tube
- Add 500 μl DF buffer; incubate at 56℃ until gel slice has been completely dissolved
- Transfer 800 μl gel mixture to DF column
- Centrifuge 13000 rpm, 1 min
- Discard the flow-through and place the DF column back
- Add 400 μl W1 buffer; Centrifuge 13000 rpm, 1 min; Discard the flow-through
- Add 600 μl wash buffer; Centrifuge 13000 rpm, 1 min; Discard the flow-through
- Centrifuge 13000 rpm, 5 min; air-dry
- Add 50 μl ddH2O; Centrifuge 13000 rpm,1 min
- Storage at –20℃
- TA ligation: alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) genes
- use pGEM®-T Easy Vector (Promega)
- 1μl pGEM®-T Easy Vector
- 5μl 2X rapid ligation buffer
- 1μl T4 DNA ligase
- 3μl insert
- 4℃, overnight
Day 8: Sep 8 ,2010
- Transformation
- transform alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) TA ligation products into XL-10 Gold competent cell
Day 9: Sep 9 ,2010
- Annealing
- anneal complementary single strain of RBS (BBa_B0034), Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) into double strain fragments
- dilute oligo primers to 10 μM/μl
- add 12.5 μl plus strain
- add 12.5 μl minus strain
- Heat to 95℃; cool down to room temperature slowly
- TA ligation
- Ligate RBS (BBa_B0034) , Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) on pGEM®-T Easy Vector (Promega)
- 1μl pGEM®-T Easy Vector
- 5μl 2X rapid ligation buffer
- 1μl T4 DNA ligase
- 3μl insert
- 4℃, overnight
- Mini-prep
- Pick alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) transformants (ligate on pGEM-T easy vector)
- Pick 4 colonies from alkB1, alkG, alkH, alkJ and GFP generator transformants
- Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg/l)); 37℃, overnight
Day10: Sep 10 ,2010
- Plasmid extraction (Homemade) : alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) transformants (ligate on pGEM-T easy vector)
- Enzyme digestion (check)
- 6 μl RNase
- 3 μl NEB buffer 4
- 0.5 μl XbaⅠ
- 0.5 μl SpeⅠ
- 10 μl plasmid DNA
- Incubate at 37℃, 3 hr
- Transformation
- transform RBS (BBa_B0034), Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) into XL-10 Gold competent cell
Day11: Sep 11 ,2010
- Mini-prep
- Pick RBS (BBa_B0034), Biobrick cloning site prefix (BBa_G00000) and Biobrick cloning site suffix (BBa_G00001) transformants (ligate on pGEM-T easy vector)
- Pick 4 colonies from RBS , Biobrick cloning site prefix and Biobrick cloning site suffix transformants
- Each colony incubate with 1.5 ml LB medium (with Ampicilin (50mg/l)); 37℃, overnight
- alkB1, alkG, alkH, alkJ and GFP generator (BBa_E0240) (have been checked in correct size and ligate on pGEM-T easy vector) mini-prep for plasmid extraction (use plasmid extraction Kit)
- 5 μl bacterial stock
- 10 ml LB (with Ampicilin (50mg/l))
- Incubate at 37℃, overnight
 "
"