Team:TU Munich/Project
From 2010.igem.org
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
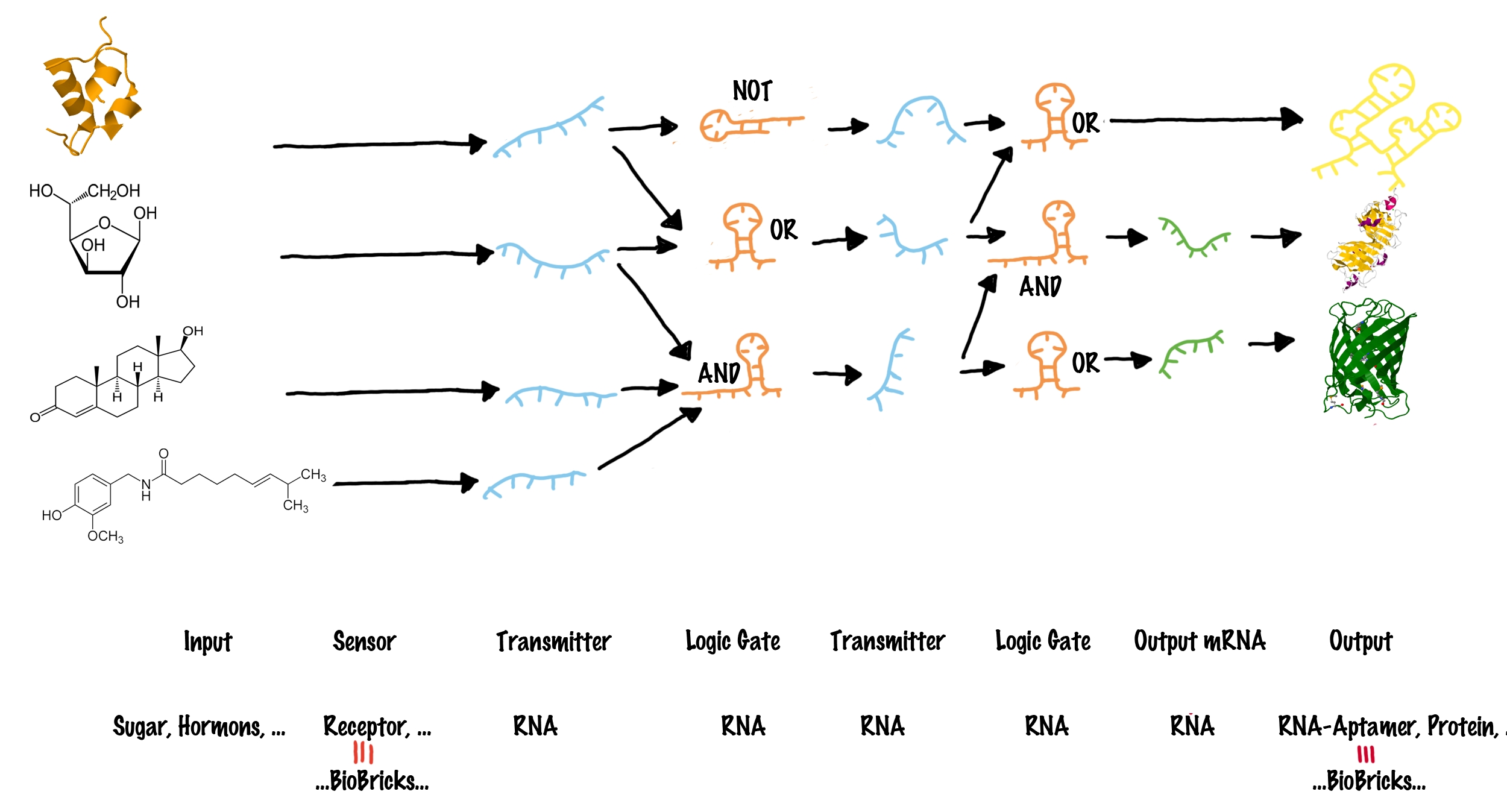
VisionUntil today, 13.628 biobrick sequences[1] have been submitted to partsregistry, thereof 102 reporter units, 12 signaling bricks and xx sensing parts.
Since there, people are trying to arrange these single biological building blocks in such a manner that allows producing special biotechnological products (metabolic engineering), developing biological sensory circuits (biosensors) and even giving microorganisms the ability to react on multiple environmental factors and serve both as disease indicator and drug. These examples and further promising ideas were implemented on previous iGEM-competitions.[2][3][4] Generally speaking, the above descirbed adapter has to meet the following requirements:
ImplementationTo functionally connect BioBricks, there are several possibilities including genetic switches, riboswitches and direct protein-protein interactions. We investigated several hypothetically principles, and decided to focus our practical work on the development of a RNA-RNA interaction-based switch. These switches are capable of changing between two states, a state of antitermination and termination, and make use of highly-specific RNA-RNA interaction. In principle such a switch can fulfill all requirements mentioned previously. The following text clarifies how these switches work in detail. How to connect BioBricksOur adapter is a system, that activates or disables BioBricks (output BioBricks) in response to the presence of other Biobricks (input Biobricks). Our approach uses a molecular network to put this into practice and consists of four major elements:
In order to connect different BioBricks, our network requires four major types of components:
Computer vs. molecular network - and our approach
Logic gates in a molecular network are often compared to transistors used in a computer, where billions of transistors are incorporated[7]. The main advantage on a computer chip is, all transistors share the same functional principle, and only the way connecting them in a special sequence allows specific addressing of only a subset of other transistors by an input. However, spatial fixed connections of molecular logic gates are not possible in a living cell. The "wiring" within a cell relies on the specific interaction between transmitter molecule and their corresponding logic gates, for example implemented by protein-protein/ligand-protein interactions or specific ligand-riboswitch interactions.[8][9] As a result, in a cell, each occurring logic gate ("transistor") has to be different, at least in a special recognition site[10] - for example like different transcription factors, recognizing different DNA-sites. Thanks to evolution, nature easily can invent a new transistor for each task - science achieves this only on a limited scale, and producing synthetic molecular logic gates artificially by either rational or evolutionary protein or riboswitch engineering, is limited to small circuits so far[11]. Our project aims to establish a real molecular transistor, which shares the same functional principle for all logic gates. At the same time, we want to design a easily exchangeable recognition site, which can individually be designed by everyone! These elements can be combined to build up a molecular network (see illustration). Each input molecule (such as a BioBrick) produces a unique transmitter molecule. All transmitters belong to the same type of molecule and share a common design. However each transmitter molecule can only interact and activate a certain subset of logic gates. In other words, logic gates have to recognize as well as bind the corresponding transmitter molecules and are capable of producing a new output transmitter molecule. Depending on the type of the logic gate (AND, OR or NOT[6]), an output transmitter is only created if both input transmitter molecules are present (AND), at least one of two input transmitters is present (OR) or if no input transmitter is present at all (NOT). Once a logic gates has produced a new output transmitter, these transmitters can in turn address another subset ("layer") of logic gates. In theory many layers of logic gates can be connected this way allowing the creation of large networks. Until this step, various transmitter molecules might have been produced. But in order to create a Biobrick output, the last layer logic gates finally generates transmitter molecules that will not active logic gates, but will rather interact with the cell metabolism to produce a BioBrick responds. In other words, the last layer of transmitter molecules is capable of regulating BioBrick formation.
Design and functional principle of logic gatesThe concept introduced above provides a framework that can potentially serve as an universal adapter between different BioBricks. However, the logic gates have not been specified more precisely so far. This will be done in the following section. Generally speaking, our logic gates are to posses the following characteristics:
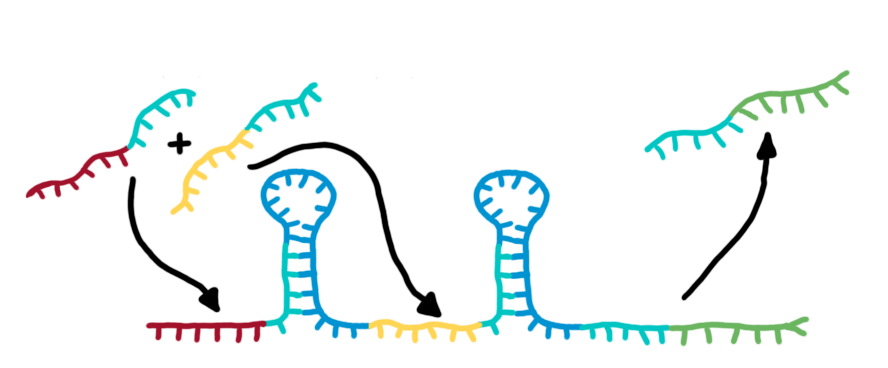
Toggle SwitchRoughly speaking, a toggle switch can be regarded as an enhance transcriptional terminator. The enhancement can be described easier by dividing a toggle switch into its components:
Summing up, the recognition site allows a specific interaction between switches and transmitter molecules. Once this interaction is formed, an interaction between the transmitter and the target will actually switch the state of the terminator. This allows the specific arrange and interconnection of numerous of these switches by transmitter molecules, without changing the target site. Comparable to wires connecting many identical transistors, our target site remains the same.
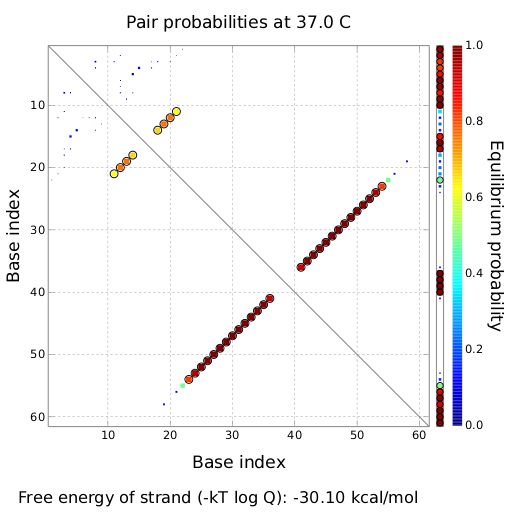
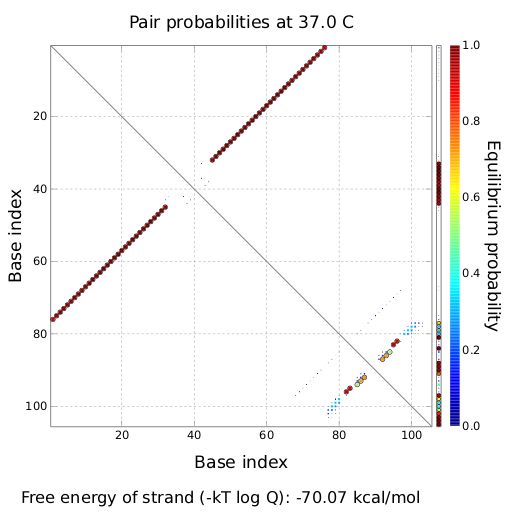
Transmitter RNA´s, input and output of bioLOGICS toggle switchesTransmitters present the "trigger" to shift switches between the "on" and "off" state. They requires the ability to change the terminators secondary structure and cause antitermination, BUT only if a special recognition site is detected. Thus, each signal consists of a trigger site, interacting with the switches trigger site and an identity site, interacting with the toggles recognition site. Practically, the shift between antitermination and termination is induced by a complementary RNA-sequence, influencing the terminators secondary structure. But in contrast to previous approaches on this field [12], we introduced the described synthetic trigger site in such a manner it is not able to change the terminator´s state on its own, but only in combination with the identity site, which is complementary to the recognition site. The challenge is to arrange and optimize these elementary building blocks thermodynamically, that a trigger site is only able to switch in combination with its respective identity site. This was done by in silico design using NUPACK, presented in section in silico design.
Putting it all together: the switching process
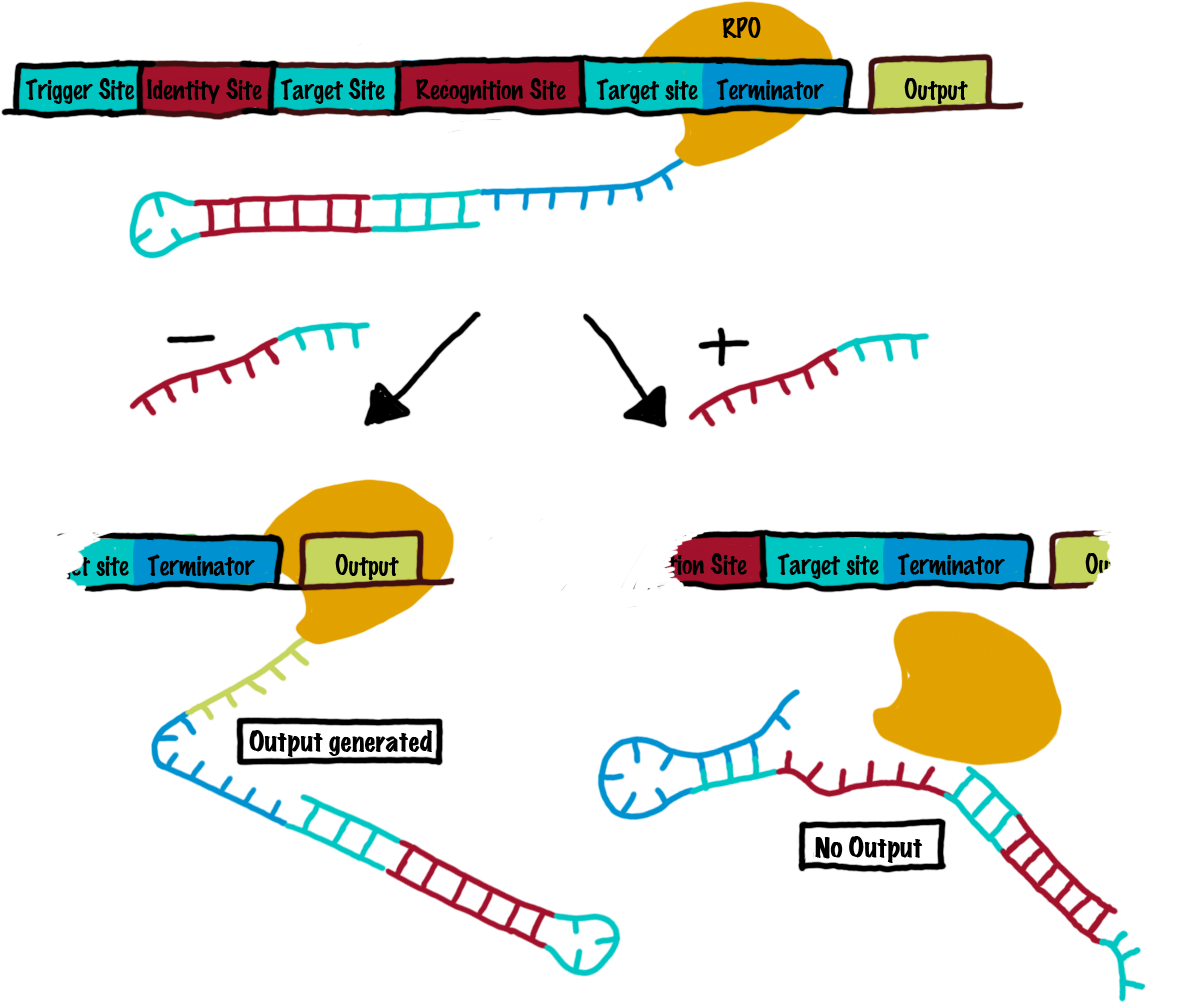
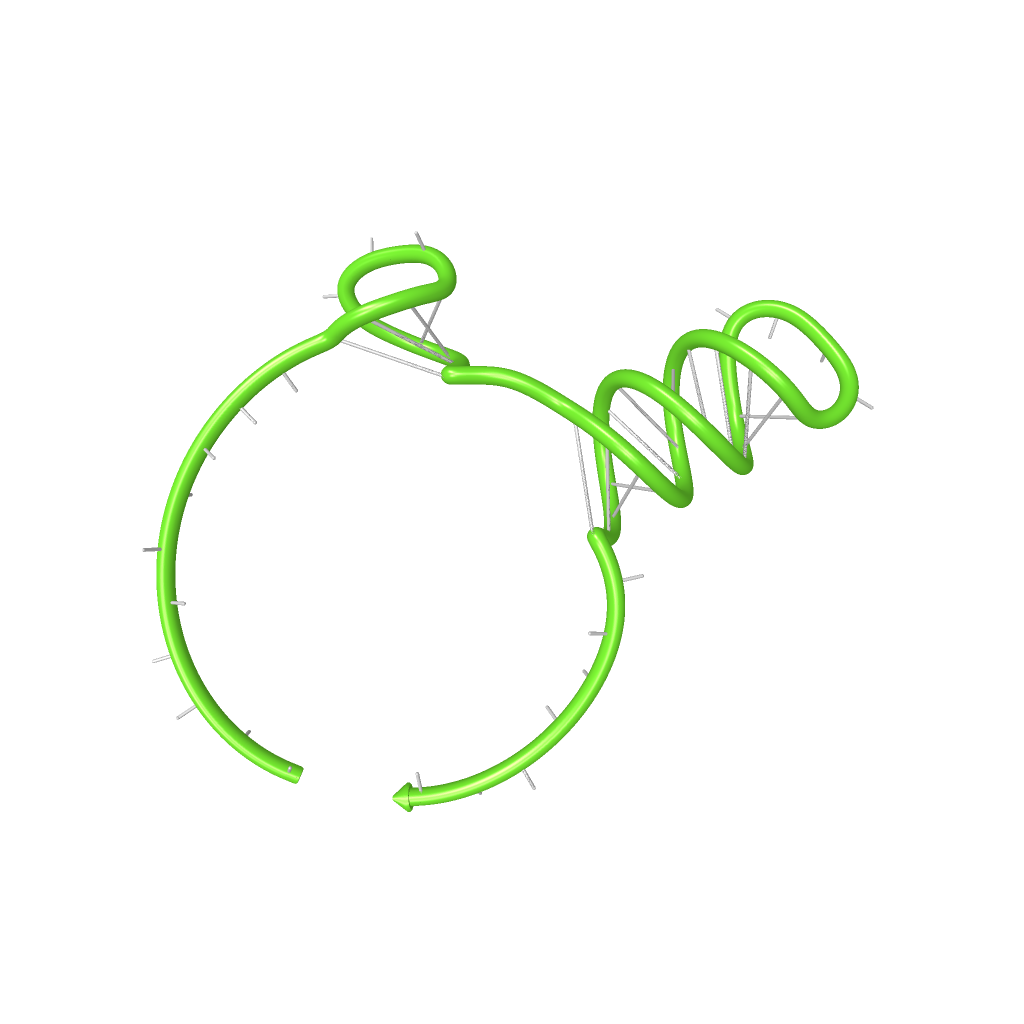
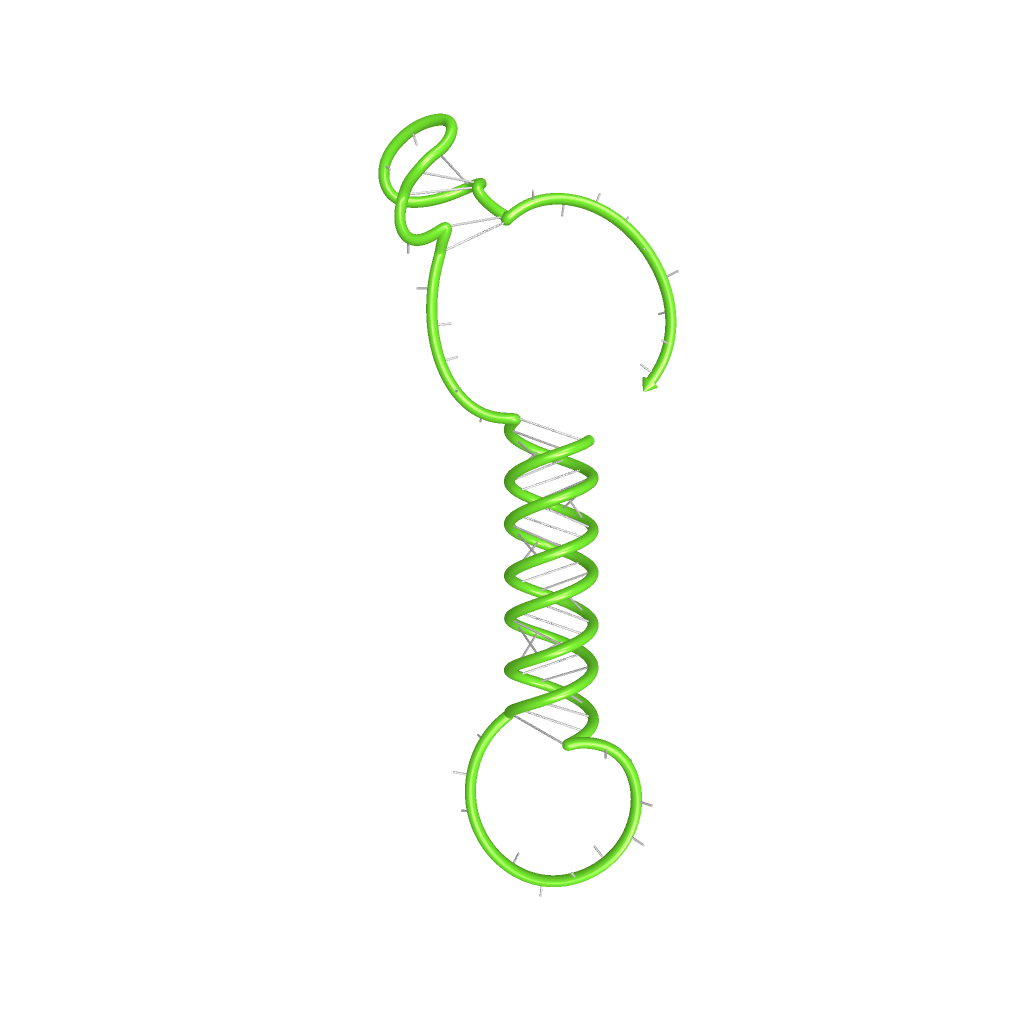
 Rectangles present the composition of our functional units on the level of DNA. Fringed lines represent RNA produced by RNA polymerase. The stem loop structure depicts the switchable terminator. Terminator and target site are illustrated in blue and turquoise, respectively. Recognition sites are indicated in different colors, in this case red for the input transmitter and green for the output transmitter.Each toggle switch and or later logical unit has to be flanked by a promotor and another constitutive terminator, to allow RNA-production by RNA-polymerase in a proper way. On the other hand, in the presence of a input transmitter, this small functional RNA inhibits the stem loop formation by complementary base-pairing and hence avoids termination of transcription. In detail, the identity site (red part on transmitter) binds the recognition site (red part on switch) and serves as toehold, which will thermodynamically allow the trigger site (turquoise part on transmitter) to perform a strand displacement and open the stem loop structure, allowing the polymerase to read through and form the output RNA.
to be competitive to terminator formation - meaning an comparison of kinetic parameters (see Modeling page)
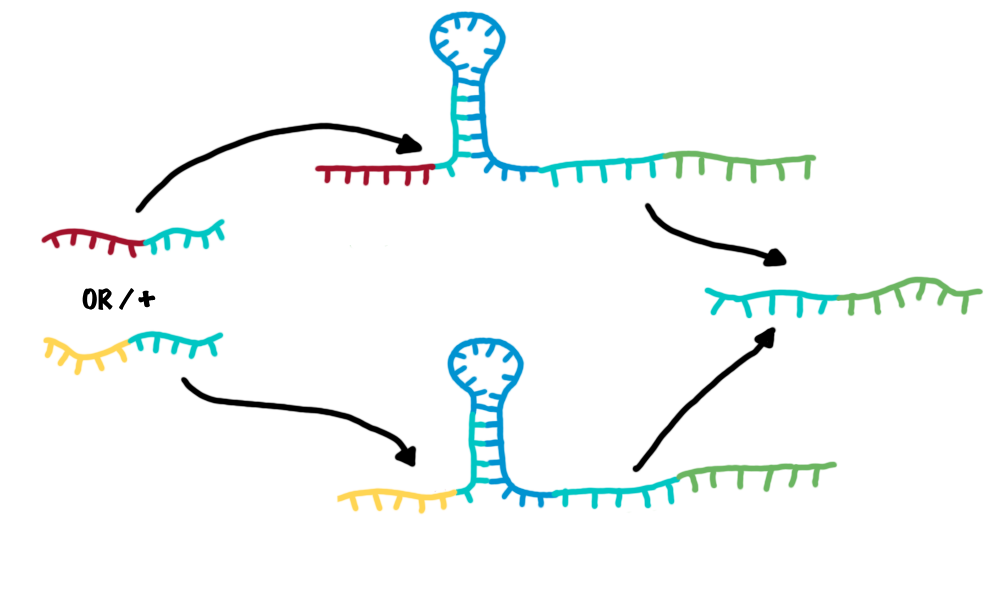
The bioLOGIC toggle switch with regard of logical operationsAs described, each switch can be accessed by a specific RNA-transmitter molecule, illustrating the input. In turn, another RNA-transmitter molecule will be produced if the switch shifts its state. This output transmitter of one switch can serve as input transmitter for the next toggle switch by meaningful selection and design of the respective recognition sites. This easily allows arranging several switches in specific sequences and faulty wiring - the corner stone of a logical network. To ease the building of logical networks, applying mathematical logics, e.g. Boolean logics like in computational science would be worthwhile. It is possible to establish general Boolean operators with our switches and thus build "logical modules". Since AND/OR/NOT are the most simple logic operations which can be implemented with the presented toggle switches, and all remaining operations can be expressed by these three operators[ZITAAAT Wiki oder so)we exemplary designed them.
Network constructionDesigning complex biological networks based on either traditional protein engineering or our new bioLOGICS is still a complex task. We developed a software which allows the fast construction of a bioLOGICS based networks.
Our ObjectivePutting the implementation described above into pratise, will be a major challenge. For this year's iGEM competition our goal is to do the first step: design and build a switch that can be toggled by a RNA molecule. To be precise, we want to modify a transcription terminator, in such a way, that it interacts with a second RNA molecule and as a result is no longer capable of forming a stem loop. Once the objective mentioned above is accomplished, these basic RNA/RNA-interactions have to be modified in such a manner that the described identity/trigger site pattern for the transmitter and the complementary recognition/target site switch composition has to be established. The most important requirement is to is to optimize these modules that the transmitter is only able to toggle switches specifically, meaning only in the presence of both, identity AND trigger site.
In silico designAs desribed above, our toggle switches are based on certain design rules. However, there still are different strucutral paramters that need to be tested and optimized (length of recognition site and target site, choice of terminator, etc.). We used in silico design and modeling ) to test different parameters. Furthermore we tried to use the antitermination principle observed in nature, such as attenuation in E. coli or tiny abortive RNA´s of T7-phage. Evaluation and MeasurementsTo evaluate the functionality of our molecular switches, we first had to establish several assays. Therefore, we improved an existing in vivo assay and developed an in vitro assay for this purpose. For more information please refer to the lab section. ResultsEvery network starts with a basic unit. While our declared aim is to enable networks allowing fine-tuning of gene expression beyond the regular on/off, exploring such an on/off switch/signal pair is the first step towards a functional network. We constructed several units and tested their efficiency, robustness and reproducibility in vivo, in vitro and in silico. Furthermore we developed a software which allows easy constructions of networks based on our designed logic gates. Conclusive elaboration of a few first RNA-based logic units is the major contribution of our iGEM team. in silico Design of Switching and Trigger Unit
attenuation principleA random sequence was derived from xxx. A complementary sequence, reaching within the terminator´s stem loop was stepwise shortened to find the length, where the formation of the terminator is thermodynamically favored compared to the strand displacement by the signal. The trigger sequence was defined by selecting the shortest unit which still is able to "destroy" the stem loop. Subsequently, the trigger unit was tested in regard of not being able to resolve the stem loop on its on. As table xxx illustrates, the terminator is thermodynamically favorite toward the trigger unit, but in combination with the specificity site, binding becomes possible.
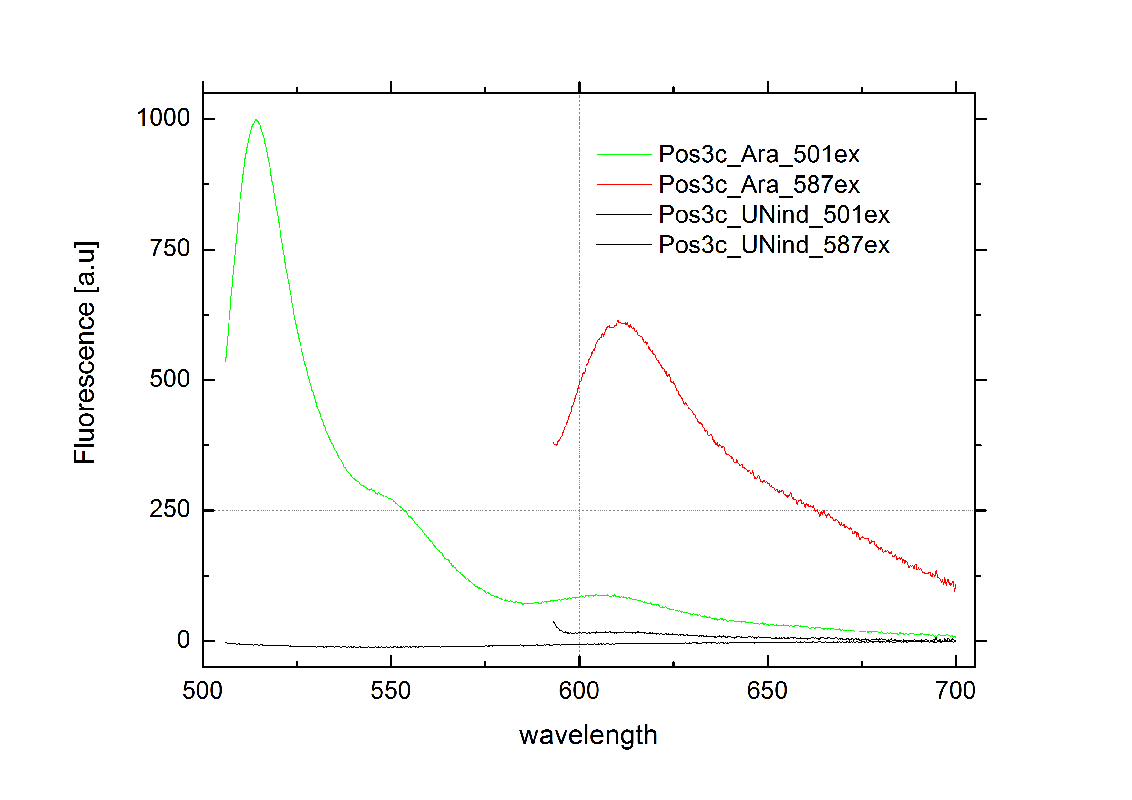
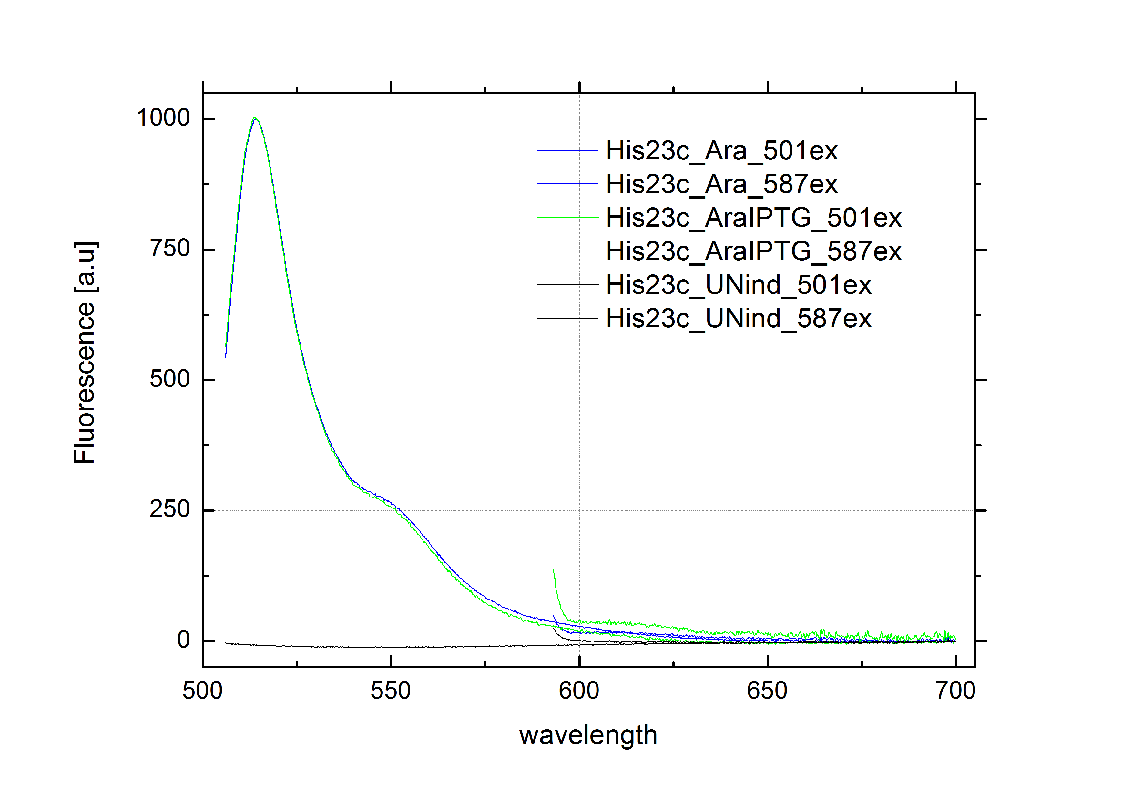
tiny abortive principleubiquitous terminatorsCloseModelingWe modeled our bioLOGICS toggle switches theoretically to prove that our they are able to interrupt termination. We also optimized the switches and the corresponding signals and furthermore took a look a combination of toggle switches that result in logic gate. See our Modeling page for further details. in vivo Functionality ScreeningSince our logic gates are intended to function in living cells, in vivo measurements were essential. In a set of experiments we concentrated on two different toggle switches based on known [[1]] from nature: the HisTerm and TrpTerm. Focusing on fluorescent proteins for quantifiable input and output we designed a functional and robust screening system. For greater detail see Experimental Design. Unfortunately, setting up a working screening system failed twice. Only in redesigning and improving the screening plasmid pSB1A10 we succeeded, but lost precious time. Ultimately, the two switches displayed remarkable differences in their terminator efficiency, but neither of them responded to their corresponding signal. However, screening one transmitter signal cannot disprove our principle system. Limited by time, we hope for future teams to take up our work and to use our improved test system that we submitted to the parts registry. Considering the high complexity of in vivo measurements compared to other experimental challenges, a robust and easy to handle test system for PoPS-based devices is desirable. As described in Experimental design, we used fluorescent proteins: eGFP to normalize the input and a RFP or mCherry to measure the output. Our first t attempt, using the screening plasmid pSB1A10 yielded no interpretable results. Switching the fluorescent protein to mCherry did not work either, but after several experimental setups we determined a transcriptional problem causing no reporter protein expression regardless of the inserted part. Thereby we demonstrated the screening plasmid pSB1A10 to be malfunctioning. Finally a new design based on pSB1A10 lead to a functional and robust screening system. A second promoter with identical induction properties inside the BioBrick cloning site enforces transcription of the PoPS-based device and the mCherry output. Exemplary, the graph below on the right shows the positive control, induced and uninduced at OD600=0.7 followed by 16 h incubation at 25 °C. Clearly visible are eGFP and mCherry fluorescence in the induced samples. The uninduced control showed no fluorescence at all, demonstrating the PBad promoter to be tight and providing no basal transcription. A major advantage for the screening system. This newly designed screening approach renders the characterization of PoPS-based devices in general and toggle switches in particular easy and robust.
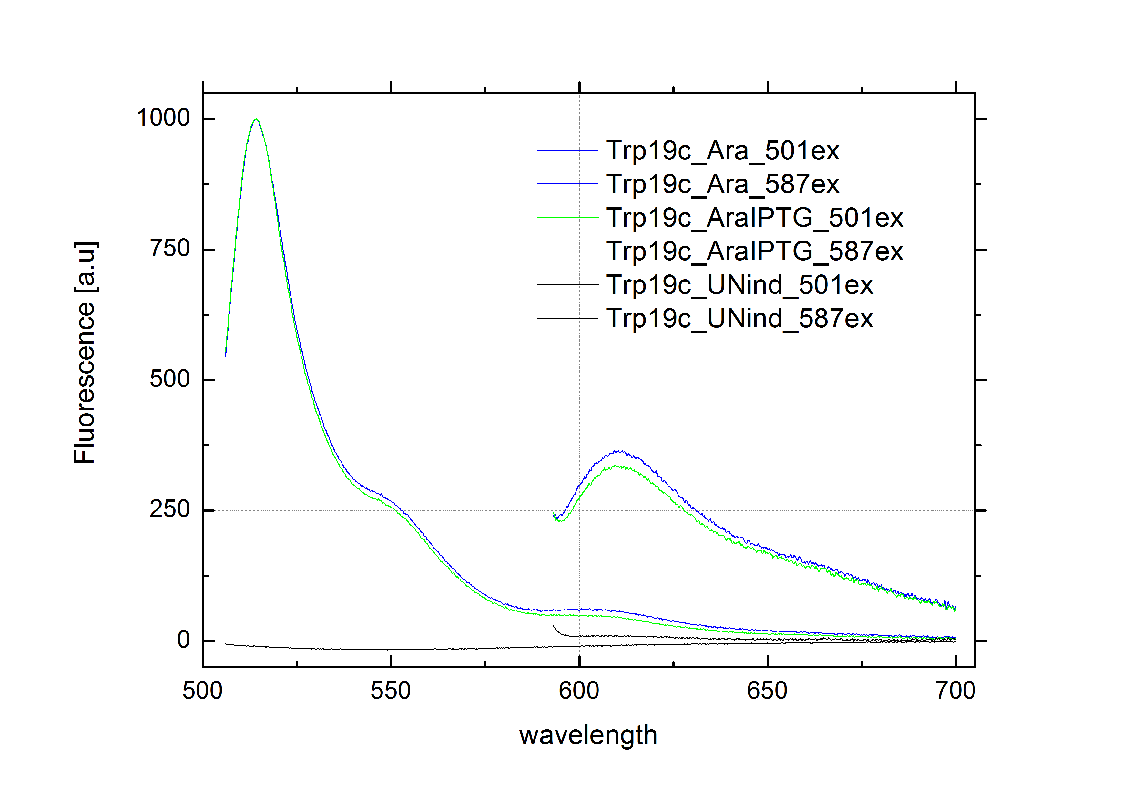
Attaining only 90% terminator effiecency, the natural Trp [[3]] is known be less effective than the HisTerm.[13] The graph on the right depicts the to our designed TrpTerm characteristic efficiency of about 40 %, notably below the natural standard. Allowing 60% transcription in the “off” state excludes the TrpTerm from possible candidates for a scalable network of logic gates. Thus the TrpTerm is inoperative as intended, but may still be useful in other contexts. Similar to the HisTerm, the TrpTerm also did not react to the induction of the corresponding signal.
Making use of our improved screening system we also carried out some in vivo kinetic measurements in addition to the end-point measurements above. In contrast to the in vitro experiments we did not obtain significant results for the chracterization of our toggle switches. As the switching process is many times faster than protein synthesis our in vivo kinetics include the synthesis of mCherry as well as its maturation. Therefore we centered our attention on end-point experiments. For more information browse the lab book. in vitro Screening
SoftwareAlthough we could not show the full functionality of bioLOGICS in the lab we still want to demonstrate the potential of our approach. Hence we implemented the idea behind our logic gates in a program which illustrates how bioLOGCIS theoretically would allow the construction of complex information processing networks interconnecting BioBricks. For further details take a look at our Software page.
Outlook... Future plans will also work with Synthetic Terminators, which will retrieve additional informations what drives the process of Termination. ... References[1] http://partsregistry.org/cgi/partsdb/Statistics.cgi [2] https://2009.igem.org/Team:Imperial_College_London/M1 encapsulation [3] https://2009.igem.org/Team:TUDelft [4] https://2008.igem.org/Team:Heidelberg [5] Smolke and so on.... [6] http://en.wikipedia.org/wiki/Logic_gate#Symbols [7] http://en.wikipedia.org/wiki/Moore's_law [8] http://en.wikipedia.org/wiki/Protein_interaction [9] http://en.wikipedia.org/wiki/Riboswitch [10] http://en.wikipedia.org/wiki/Binding_sites + http://en.wikipedia.org/wiki/Recognition_site [11] irgend ein damn review über directed evolution and so on [12] Delorme, Ehrlich and Renault, Regulation of Expression of the Lactococcus lactis Histidine Operon. Journal of Bacteriology, Apr. 1999, p. 2026–2037 [13] Trun and Trempy(2003): Fundamental Bacterial Genetics, Wiley-Blackwell, Chapter 12 [14]Sooncheol Lee, Huong Minh Nguyen and Changwon Kang, Tiny abortive initiation transcripts exert antitermination activity on an RNA hairpin-dependent intrinsic terminator. Nucleic Acids Research, 2010, 1–9 [15] [16]
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"