Team:TU Munich/Project
From 2010.igem.org
Hartlmueller (Talk | contribs) |
(→Impementation) |
||
| Line 22: | Line 22: | ||
:The adapter is supposed to not only associate different BioBricks, but to functionally connect BioBricks in a precisely determined manner (including operations such as AND/OR/NOT). | :The adapter is supposed to not only associate different BioBricks, but to functionally connect BioBricks in a precisely determined manner (including operations such as AND/OR/NOT). | ||
<br> | <br> | ||
| - | = | + | =Implementation= |
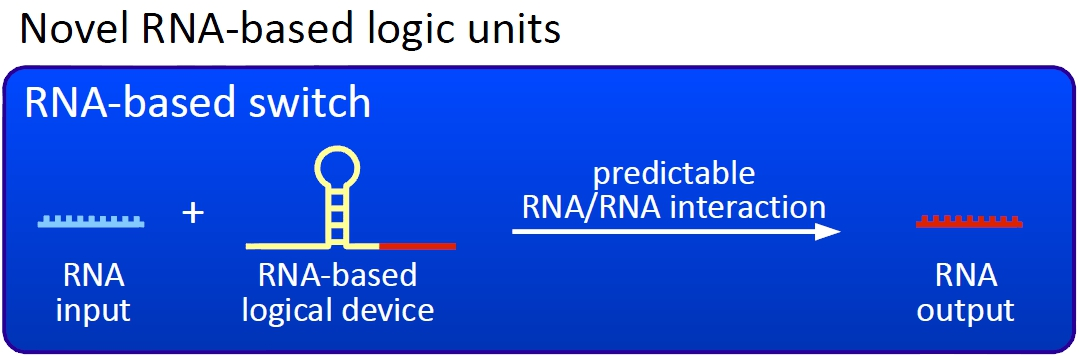
Several biological logic units, devices and circuits have been developed so far<sup>[[Team:TU_Munich/Project#ref1|[5]]]</sup>, but to our knowledge, none of them provides the opportunity to be a universal smallest unit like a transistor is in computational science. We investigated several hypothetically principles, and decided to focus our practical work on the development of a RNA-RNA interaction-based transistor by implementing two approaches: | Several biological logic units, devices and circuits have been developed so far<sup>[[Team:TU_Munich/Project#ref1|[5]]]</sup>, but to our knowledge, none of them provides the opportunity to be a universal smallest unit like a transistor is in computational science. We investigated several hypothetically principles, and decided to focus our practical work on the development of a RNA-RNA interaction-based transistor by implementing two approaches: | ||
Revision as of 08:26, 21 October 2010
|
||||||||
|
|
VisionUntil today, 13.628 biobrick sequences[1] have been submitted to partsregistry, thereof 102 reporter units, 12 signaling bricks and xx sensing parts.
Since there, people are trying to arrange these single biological building blocks in such a manner that allows producing special biotechnological products (metabolic engineering), developing biological sensory circuits (biosensors) and even giving microorganisms the ability to react on multiple environmental factors and serve both as disease indicator and drug. These examples and further promising ideas were implemented on previous iGEM-competitions.[2][3][4]
ImplementationSeveral biological logic units, devices and circuits have been developed so far[5], but to our knowledge, none of them provides the opportunity to be a universal smallest unit like a transistor is in computational science. We investigated several hypothetically principles, and decided to focus our practical work on the development of a RNA-RNA interaction-based transistor by implementing two approaches:
Reference[1] http://partsregistry.org/cgi/partsdb/Statistics.cgi [2] https://2009.igem.org/Team:Imperial_College_London/M1 encapsulation [3] https://2009.igem.org/Team:TUDelft [4] https://2008.igem.org/Team:Heidelberg [5] Smolke and so on....
ConceptThe basic principle of our switches are short RNA sequences, the scientific idea shares similiarities with the principle of antitermination but also inherits a completely new way of RNA based transcription regulation. We used three-dimensional structure predictions and thermodynamic calculation to develop a set of switches - about 50 nucleotides - and signals - about 20 nucleotides. The switch forms a stem loop causing transcription termination, which can be resolved upon binding of a signal. On/off switching can therefore be easily controlled by signal avaiability and provides a new concept to control gene transcription.
Our switches are based on the principle of transcriptional antitermination. Transcription can be cancelled by the formation of a RNA stem loop in the nascent RNA-chain, which then causes the RNA polymerase to stop transcription and fall off the DNA. We use sequences as transcriptional switches, that are calculated to be capable of stem loop formation. We plan to control the termination at these switches with a small RNA molecule (our RNA "signal") that is complementary to a part of the stem loop forming sequence. This small functional RNA inhibits the stem loop formation by complementary base-pairing and hence avoids transcription termination. The signal is composed of two parts: While the first part provides specifity (recognition site), the second part causing stem loop disintegration (functional core) can in principle be the same for all bioLOGICS. Therefore variation of the first part allows the construction of an endless number of switches. The functional core causing stem loop disintegration is based on a working system (see attenuation) established by nature. Different stem loops were tested in this effort: Regulatory parts from the E. coli trp-operon, his-operon and one based on previous iGEM-work.
Network constructionDesigning complex biological networks based on either traditional protein engineering or our new bioLOGICS is still a complex task. We developed a software which allows the fast construction of a bioLOGICS based networks. Work ProgressEvery network starts with a basic unit. While our declared aim is to enable networks allowing fine-tuning of gene expression beyond the regular on/off, exploring such an on/off switch/signal pair is the first step towards a functional network. We constructed several units and tested their efficiency, robustness and reproducibility in vivo, in vitro and in silico. Furthermore we developed a software which allows easy constructions of networks delivering a ready network. Conclusive elaboration of a few first RNA-based logic units is the major contribution of our iGEM team. Results |
|||||||
 "
"